PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1849938

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1849938
In Vitro Diagnostic - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
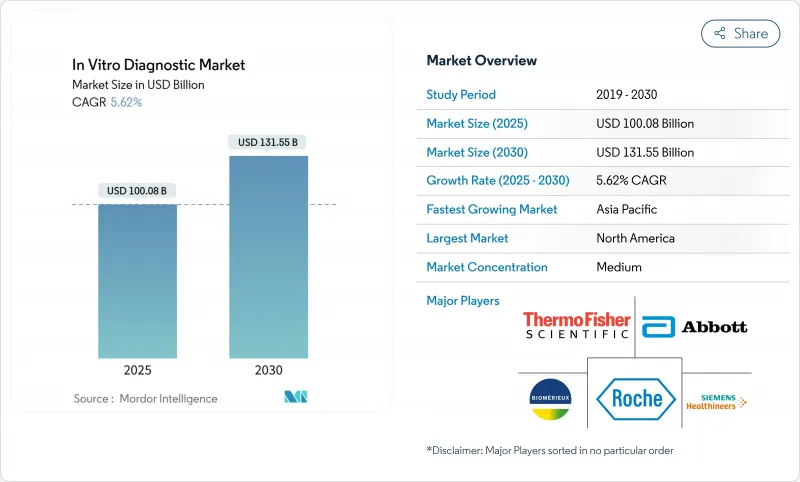
The in vitro diagnostics market stood at USD 100.08 billion in 2025 and is forecast to reach USD 131.55 billion by 2030, advancing at a 5.62% CAGR.
Expansion is propelled by AI-enabled pathology, platform automation, and rapid point-of-care (POC) technologies that compress turnaround times and broaden access to high-value testing. Intensifying chronic disease burdens, an aging population, and payer support for early detection sustain steady test volume growth, while software-centric innovations unlock data-driven clinical decisions. Consolidation has accelerated as incumbents purchase niche innovators to secure next-generation capabilities, though supply-chain fragilities, from enzyme shortages to hurricane-related IV-fluid disruptions, highlight operational risks that shape sourcing strategies. Regulatory shifts run in parallel: the FDA's laboratory-developed test rule raises compliance costs even as Europe's IVDR transition extensions delay, not remove, standardization pressures.
Global In Vitro Diagnostic Market Trends and Insights
High Prevalence of Chronic Diseases
Seventy-six percent of US adults reported at least one chronic ailment in 2024, driving sustained diagnostic demand in diabetes, cardiovascular, oncology, and nephrology care. Frequent monitoring aligns with payer incentives for early intervention that trims downstream treatment costs. Laboratories respond by introducing assays for NGAL, cystatin C, and KIM-1 that flag kidney injury before symptoms appear. AI models now triage longitudinal result patterns to predict exacerbations earlier, while automation sustains throughput amid workforce shortages. These dynamics establish the in vitro diagnostics market as an indispensable pillar of chronic-care pathways.
Expanding Adoption of Point-of-Care Diagnostics
POC platforms have evolved from single-analyte strips to multiplex molecular systems that deliver laboratory-grade results in under 15 minutes. Roche's 12-target respiratory PCR and Dragonfly's portable mpox test exemplify this leap, pairing sensitivity above 95% with field portability. Retail clinics are mainstreaming such tools: CVS now offers 3-in-1 flu-COVID panels across 1,600 sites, broadening access while easing hospital load. For payers, every minute saved in acute infection management curbs transmission risk and costly admissions, reinforcing POC economics. Consequently, penetration in Asia-Pacific primary-care chains accelerates overall in vitro diagnostics market growth.
Stringent Multi-Region Regulatory Approval Timelines
The FDA's laboratory-developed test rule imposes up to USD 3.56 billion in new compliance costs, stretching smaller innovators' budgets and delaying product launches. Europe's IVDR extension buys time, yet manufacturers must still upgrade quality systems and secure notified-body slots, a resource bottleneck that inflates timelines. Dual submissions across jurisdictions fragment strategies and divert R&D spend away from new assay exploration. The drag is acute for AI-based tools that lack historical precedents, complicating dossier preparation and reviewer training, thereby tempering the overall in vitro diagnostics market trajectory.
Other drivers and restraints analyzed in the detailed report include:
- Continuous Platform Innovation (AI, Automation, Multiplexing)
- Growing Acceptance of Personalized / Companion Diagnostics
- Reimbursement Uncertainty Across Emerging Test Classes
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Immunodiagnostics captured 29.05% of in vitro diagnostics market share in 2024, anchored by protein assays indispensable to infectious disease and metabolic testing. Yet molecular diagnostics is forecast to grow 6.59% annually, lifting the segment's in vitro diagnostics market size as next-generation sequencing, multiplex PCR, and isothermal amplification become mainstays of precision medicine. Roche's temperature-triggered 12-pathogen PCR further compresses run-times, removing throughput constraints that once limited molecular adoption in decentralized settings.
Cross-fertilization is rising: hybrid platforms detect nucleic acids and antigens in the same cartridge, blending molecular specificity with immunoassay ease. AI algorithms stitch gene-expression results with serological markers, refining prognostic accuracy in oncology and antimicrobial stewardship. Laboratories thus migrate workloads from legacy immunoassays to low-cost, high-plex nucleic-acid formats, sustaining molecular's outperformance through 2030.
Reagents represented 55.35% of in vitro diagnostics market size in 2024, reflecting repeat-purchase economics of consumables. However, software and services are growing at 9.35% CAGR as laboratories invest in AI analytics that unlock workflow efficiencies and predictive insights. Philips and Ibex reported 37% productivity gains when digital pathology AI triaged slides before pathologist review.
Subscription models convert lumpy analyzer sales into recurring revenue, aligning vendor incentives with outcome-based performance. Cloud-native platforms enable overnight algorithm upgrades and multi-site data pooling for continuous learning. As a result, reagent leaders are bundling algorithm licenses with kit sales, cementing customer lock-in across the in vitro diagnostics market.
The in Vitro Diagnostics Market Report is Segmented by Test Type (Clinical Chemistry, Molecular Diagnostics, and More), Product (Instruments, Reagents and More), Usability (Disposable IVD Devices, Reusable Equipment), Application (Infectious Diseases, Oncology, Cardiology, and More), End User (Stand-Alone Laboratories, and More), Geography (North America, Europe, and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America retained 38.08% in vitro diagnostics market share in 2024 on the back of robust reimbursement, entrenched R&D, and pioneering AI deployments. The FDA's LDT rule, while costly, could lift quality standards and foster nationwide data interoperability if harmonized across states. Retail health continues to redefine access; CVS and Walgreens deploy POC molecular panels that shorten diagnostic journeys and create new volume streams. Meanwhile, supply-chain shocks, BD culture-media shortages and Baxter IV-fluid disruptions, have prompted renewed investment in domestic manufacturing resiliency.
Asia-Pacific is the fastest-growing region at 6.85% CAGR, propelled by demographic aging and government funding expansions in Japan, China, and India. Labcorp's extended tie-up with BML Japan and Sansure's China joint venture for sepsis assays illustrate cross-border technology transfer that accelerates precision medicine uptake. Domestic Chinese firms are scaling cost-efficient analyzers for export, leveraging experience from vast local screening initiatives. These moves collectively enlarge regional in vitro diagnostics market capacity and foster competitive pricing pressures globally.
Europe posts steady gains despite IVDR turbulence. Transition delays prevent immediate test shortages, yet long-term harmonization is unavoidable, compelling smaller manufacturers to seek strategic partners or exit. The continent commands leadership in spatial-omics and digital pathology, evidenced by Philips-Ibex's AI edge and Diagnexia's prostate AI partnership that shortens pathology turnaround. Middle East, Africa, and Latin America build capacity mainly through POC and drug-screening programs, as Intelligent Bio's collaboration with IVY Diagnostics expands distribution networks that bridge regulatory and cultural gaps.
- Roche
- Abbott Laboratories
- Siemens Healthineers
- Danaher Corp (Beckman Coulter, Cepheid)
- Thermo Fisher Scientific
- Sysmex Corp
- bioMerieux
- Beckton Dickinson
- Bio-Rad Laboratories
- QIAGEN
- DiaSorin
- Grifols
- Agilent Technologies
- Ortho Clinical Diagnostics / QuidelOrtho
- Hologic
- Illumina
- PerkinElmer
- Randox Laboratories
- Meril Diagnostics Pvt Ltd
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 High Prevalence Of Chronic Diseases
- 4.2.2 Expanding Adoption Of Point-Of-Care (POC) Diagnostics
- 4.2.3 Continuous Platform Innovation (AI, Automation, Multiplexing)
- 4.2.4 Growing Acceptance Of Personalized / Companion Diagnostics
- 4.2.5 Retail-Clinic & At-Home Sampling Ecosystems
- 4.2.6 Convergence Of Spatial-Omics & IVD Workflows
- 4.3 Market Restraints
- 4.3.1 Stringent Multi-Region Regulatory Approval Timelines
- 4.3.2 Reimbursement Uncertainty Across Emerging Test Classes
- 4.3.3 Cyber-Security & Data-Interoperability Gaps In Connected IVD
- 4.3.4 Enzyme / Reagent Supply-Chain Exposure To Geo-Political Export Controls
- 4.4 Supply-Chain Analysis
- 4.5 Technological Outlook
- 4.6 Porter's Five Forces
- 4.6.1 Threat of New Entrants
- 4.6.2 Bargaining Power of Buyers
- 4.6.3 Bargaining Power of Suppliers
- 4.6.4 Threat of Substitutes
- 4.6.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Test Type
- 5.1.1 Clinical Chemistry
- 5.1.2 Immunodiagnostics
- 5.1.3 Molecular Diagnostics
- 5.1.4 Hematology
- 5.1.5 Coagulation
- 5.1.6 Microbiology
- 5.1.7 Other Test Types
- 5.2 By Product
- 5.2.1 Instruments
- 5.2.2 Reagents & Kits
- 5.2.3 Software & Services
- 5.3 By Usability
- 5.3.1 Disposable IVD Devices
- 5.3.2 Re-usable Equipment
- 5.4 By Application
- 5.4.1 Infectious Diseases
- 5.4.2 Diabetes
- 5.4.3 Oncology
- 5.4.4 Cardiology
- 5.4.5 Auto-immune Disorders
- 5.4.6 Nephrology
- 5.4.7 Other Applications
- 5.5 By End User
- 5.5.1 Stand-alone Laboratories
- 5.5.2 Hospital-based Laboratories
- 5.5.3 Point-of-Care Settings
- 5.5.4 Home-care & Self-testing Users
- 5.6 Geography
- 5.6.1 North America
- 5.6.1.1 United States
- 5.6.1.2 Canada
- 5.6.1.3 Mexico
- 5.6.2 Europe
- 5.6.2.1 Germany
- 5.6.2.2 United Kingdom
- 5.6.2.3 France
- 5.6.2.4 Italy
- 5.6.2.5 Spain
- 5.6.2.6 Rest of Europe
- 5.6.3 Asia-Pacific
- 5.6.3.1 China
- 5.6.3.2 Japan
- 5.6.3.3 India
- 5.6.3.4 South Korea
- 5.6.3.5 Australia
- 5.6.3.6 Rest of Asia-Pacific
- 5.6.4 Middle East and Africa
- 5.6.4.1 GCC
- 5.6.4.2 South Africa
- 5.6.4.3 Rest of Middle East and Africa
- 5.6.5 South America
- 5.6.5.1 Brazil
- 5.6.5.2 Argentina
- 5.6.5.3 Rest of South America
- 5.6.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Business Segments, Financials, Headcount, Key Information, Market Rank, Market Share, Products and Services, and analysis of Recent Developments)
- 6.3.1 F. Hoffmann-La Roche Ltd
- 6.3.2 Abbott Laboratories
- 6.3.3 Siemens Healthineers AG
- 6.3.4 Danaher Corp (Beckman Coulter, Cepheid)
- 6.3.5 Thermo Fisher Scientific Inc.
- 6.3.6 Sysmex Corp
- 6.3.7 bioMerieux SA
- 6.3.8 Becton, Dickinson and Company
- 6.3.9 Bio-Rad Laboratories Inc.
- 6.3.10 Qiagen NV
- 6.3.11 DiaSorin SpA
- 6.3.12 Grifols SA
- 6.3.13 Agilent Technologies Inc.
- 6.3.14 Ortho Clinical Diagnostics / QuidelOrtho
- 6.3.15 Hologic Inc.
- 6.3.16 Illumina Inc.
- 6.3.17 PerkinElmer Inc.
- 6.3.18 Randox Laboratories Ltd
- 6.3.19 Meril Diagnostics Pvt Ltd
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-need Assessment




