PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1850204

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1850204
Sample Preparation - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The global sample preparation market, valued at USD 9.46 billion in 2025, is projected to grow to USD 11.71 billion by 2030, reflecting a steady expansion at a 4.35% CAGR.
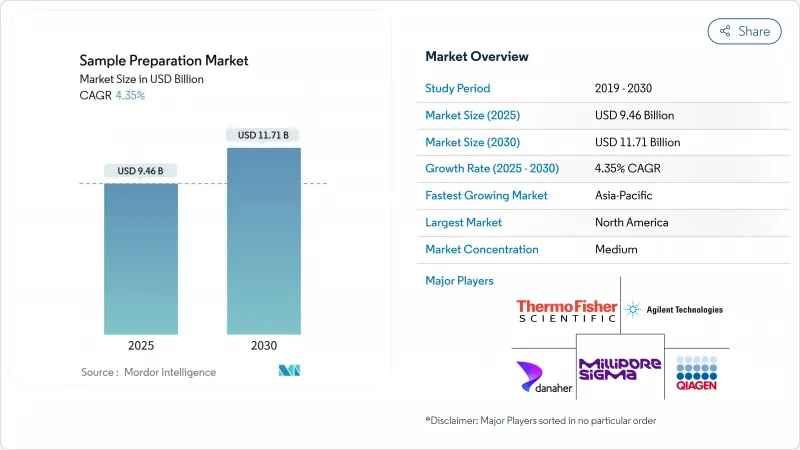
The market is driven by sustained investments in omics research, stricter data-quality standards, and increased adoption of automated, high-throughput instruments in clinical and pharmaceutical laboratories. By 2030, fully automated platforms are expected to capture a significant share of the market as laboratories address workforce shortages while striving for enhanced reproducibility. The growing implementation of precision-medicine programs in secondary and tertiary care settings is further fueling the demand for standardized upstream processing of genomic, proteomic, and metabolomic specimens. This shift is strengthening the influence of consumables vendors, whose proprietary chemistries ensure recurring revenue streams despite extended instrument replacement cycles. Additionally, regional trends indicate that the Asia Pacific market is steadily narrowing the gap with established players, supported by domestic innovation policies and the strategic relocation of biomanufacturing capacities.
Global Sample Preparation Market Trends and Insights
Surging Global Investment in Omics Research and Precision-Medicine
The integration of multi-omics data into electronic medical recordsis driving significant advancements in data harmonization, fueling the demand for sophisticated sample preparation technologies. Hospitals deploying unified genomics and proteomics dashboards have pinpointed sample inconsistencies as a critical factor causing analytical variances. To address this challenge, they are increasingly adopting validated, kit-based workflows that standardize extraction efficiencies across various specimen types, ensuring reliable results and enabling the identification of actionable biomarkers. This shift is propelling the sample preparation market, with vendors striving to certify their chemistries for compatibility with both next-generation sequencing and high-resolution mass spectrometry platforms. The growing overlap between clinical and research laboratories, driven by shared requirements for traceability, is reshaping market dynamics. In response, regulatory bodies are updating guidelines on pre-analytical variables, creating additional compliance challenges and raising the entry barriers for new players in the market.
Growing Demand for Automated, High-Throughput Sample-Prep to Boost Laboratory Productivity
In response to rising test volumes and staffing shortages, laboratories are increasingly investing in automated liquid-handling stations capable of processing 96- or 384-well plates in under an hour. Automation is proving to be a strategic asset, delivering a 1.8-fold reduction in sample-to-sample variation for proteomics workflows, thereby enhancing both quality and productivity. This trend is driving growth in the Sample Preparation market, with hardware OEMs and reagent specialists forming strategic partnerships to offer turnkey solutions that reduce the validation burden for end users. Early adopters report that reallocating technicians from repetitive pipetting tasks to data interpretation not only improves workforce morale but also shortens report-generation timelines, a critical competitive advantage in the contract-research market. Consequently, procurement committees are adopting a broader valuation framework, assessing return on investment not only through throughput metrics but also by factoring in opportunity cost savings. This evolving perspective is accelerating the shift from semi-automated to fully automated platforms, further driving market adoption.
High Capital and Operating Costs of Fully Automated Sample-Prep Platforms
High costs of comprehensive workstations, often exceeding USD 100,000, create a significant barrier for small laboratories and price-sensitive regions, impacting market adoption. Additionally, annual operating expenditures, including service contracts, calibrations, and proprietary consumables, account for 15% to 20% of the workstation's list price, driving laboratories to adopt cautious budgeting strategies. This cost dynamic has segmented the Sample Preparation market: high-throughput reference centers justify investments in premium machines, while community hospitals prefer modular systems or reagent-rental models to optimize cost efficiency. In response, vendors are strategically launching scalable systems with core decks designed to accommodate optional modules. These systems allow laboratories to add magnetic-bead or vacuum-filtration units as demand grows, aligning with evolving operational needs. This modularity not only extends asset lifecycles but also reduces the risk of technological obsolescence, enhancing resale values in the secondary-equipment market and strengthening market competitiveness.
Other drivers and restraints analyzed in the detailed report include:
- Increasing Clinical Adoption of Genomic Sequencing and Liquid-Biopsy Diagnostics
- Expansion of Biopharma R&D and Manufacturing Volumes Requiring Robust Sample Preparation
- Shortage of Skilled Personnel to Operate and Maintain Sophisticated Systems
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
In 2024, consumables dominated the sample preparation market, securing a 54.1% share and emerging as the top revenue source for vendors. This stronghold is attributed to consistent repurchase cycles; each test relies on extraction columns, beads, or buffer kits, ensuring stable cash flows that remain unaffected by capital-equipment cycles. Within the consumables realm, sample-prep kits are projected to grow at a 9.1% CAGR from 2025 to 2030, surpassing the growth of general reagents. This shift is driven by laboratories' preference for method-validated kits, which reduce inter-operator variability. The trend is especially evident in liquid-biopsy workflows, where kits designed for cell-free DNA extraction achieve superior recovery from minimal plasma volumes. Furthermore, once a laboratory commits to a proprietary chemistry linked to specific instrumentation, it gains enhanced pricing power over consumables. This trend motivates vendors to create cartridges and columns that are either physically or electronically compatible with their platforms, bolstering customer loyalty.
In 2024, semi-automated technologies held a 47.2% share of the sample preparation market, appealing to labs that seek moderate throughput improvements without overhauling their operations. These systems often integrate bench-top magnetic-bead processors with manual pipetting stations, striking a balance between cost and performance. However, fully automated platforms are projected to grow at a 10.4% CAGR through 2030, fueled by escalating labor costs and stringent reproducibility standards. Labs that have embraced fully automated solutions frequently highlight added advantages, including enhanced traceability and reduced cross-contamination, both of which diminish expensive re-runs. Furthermore, as software updates roll out new protocols remotely, the longevity of automation hardware increases, making it more appealing for budget committees assessing the total cost of ownership.
The Sample Preparation Market is Segmented by Product (Sample-Preparation Instruments, Consumables, and More), Technology (Manual, Semi-Automated, and More), Application (Genomics, Proteomics, Epigenomics, and More), End-User (Pharmaceutical Companies, Biotechnology Companies, and More), and Geography (North America, Europe, Asia-Pacific, and More). The Market Sizes and Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America leads the Sample Preparation market with 35.4% market share, buoyed by strong federal research grants, swift adoption of next-gen lab automation, and a concentration of biopharma headquarters. The region's regulatory framework, guided by FDA and CLIA standards, enforces pre-analytical quality controls, driving demand for standardized kits and traceable workflows. Partnerships, like QIAGEN's with Bio-Manguinhos/Fiocruz, highlight established vendors' efforts to adapt North American solutions for emerging public health markets, enhancing global presence and tailoring products to diverse resources. Consequently, academic medical centers, seeking distinction, are channeling investments into single-cell multi-omics platforms. This boosts consumables throughput, even as instrument installations plateau. The regional market leans towards integrated, compliance-focused solutions, streamlining documentation for accreditation cycles.
Asia Pacific is witnessing the fastest growth, driven by a surge in pharmaceutical manufacturing and robust government incentives bolstering domestic biotech ecosystems. China's Five-Year Plans allocate significant funds towards high-end instrumentation, pushing local labs to bypass intermediate technologies in favor of fully automated workflows. In Japan and South Korea, an ageing population is driving up the demand for molecular-diagnostic testing, particularly in oncology and inherited disorders. The rise of local-language software and smaller reagent pack sizes underscores the potential of regional customization in capturing market share, all while keeping core chemistries intact. Notably, recent geopolitical disruptions have underscored the importance of supply-chain resiliency, prompting multinationals to set up manufacturing hubs in the region to secure tenders.
Europe's Sample Preparation industry thrives on initiatives like Horizon Europe, channeling funds into omics projects that demand meticulous sample handling. EU mandates on lab sustainability spark a shift towards eco-friendly consumables, urging vendors to innovate kits that minimize environmental harm while maximizing yield. The rise of academic-industrial partnerships accelerates the development of specialized extraction chemistries, birthing start-ups that flourish through licensing agreements with industry giants. Concurrently, the stringent data-protection mandates of GDPR heighten the demand for secure, audit-compliant instrument software, reshaping procurement decisions alongside traditional performance benchmarks. Collectively, these dynamics reinforce Europe's pivotal role as a trendsetter in global regulatory and sustainability movements.
- Thermo Fisher Scientific
- Agilent Technologies
- Merck KGaA (MilliporeSigma)
- Danaher Corporation (Beckman Coulter & Cytiva)
- QIAGEN
- PerkinElmer
- Illumina
- Bio-Rad Laboratories
- Tecan Group
- Biotage
- Norgen Biotek
- Eppendorf
- Hamilton Company
- Promega
- LGC Biosearch Technologies
- Takara Bio
- Beckton Dickinson
- Fluidigm
- Analytik Jena GmbH
- Brooks Life Sciences
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Surging Global Investment in Omics Research and Precision-Medicine
- 4.2.2 Growing Demand for Automated, High-Throughput Sample-Prep to Boost Laboratory Productivity
- 4.2.3 Increasing Clinical Adoption of Genomic Sequencing and Liquid-Biopsy Diagnostics
- 4.2.4 Expansion Of Biopharma R&D And Manufacturing Volumes Requiring Robust Sample Preparation
- 4.2.5 Supportive Government Funding and Public-Private Partnerships for Life-Science Tool Innovation
- 4.2.6 Advancements In Automation, Microfluidics and Reagent Chemistries Enhancing Workflow Efficiency
- 4.3 Market Restraints
- 4.3.1 High Capital and Operating Costs of Fully Automated Sample-Prep Platforms
- 4.3.2 Shortage Of Skilled Personnel to Operate and Maintain Sophisticated Systems
- 4.3.3 Stringent Regulatory Requirements for Clinical-Grade Reagents Extending Time-To-Market
- 4.3.4 Supply-Chain Vulnerabilities for Specialty Enzymes, Magnetic Beads and Plastics
- 4.4 Regulatory Outlook
- 4.5 Technological Outlook
- 4.6 Porter's Five Forces Analysis
- 4.6.1 Threat of New Entrants
- 4.6.2 Bargaining Power of Buyers
- 4.6.3 Bargaining Power of Suppliers
- 4.6.4 Threat of Substitute Products
- 4.6.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Product
- 5.1.1 Sample-Preparation Instruments
- 5.1.1.1 Extraction Systems
- 5.1.1.2 Automated Workstations
- 5.1.1.3 Evaporation Systems
- 5.1.1.4 Liquid-Handling Platforms
- 5.1.1.5 Other Instruments
- 5.1.2 Consumables
- 5.1.3 Sample-Preparation Kits
- 5.1.3.1 Purification Kits
- 5.1.3.2 Isolation Kits
- 5.1.3.3 Extraction Kits
- 5.1.4 Accessories & Software
- 5.1.1 Sample-Preparation Instruments
- 5.2 By Technology
- 5.2.1 Manual
- 5.2.2 Semi-Automated
- 5.2.3 Fully Automated
- 5.3 By Application
- 5.3.1 Genomics
- 5.3.2 Proteomics
- 5.3.3 Epigenomics
- 5.3.4 Other Application
- 5.4 By End-User
- 5.4.1 Pharmaceutical Companies
- 5.4.2 Biotechnology Companies
- 5.4.3 Molecular Diagnostics Labs
- 5.4.4 Academic & Research Institutes
- 5.4.5 CROs & CDMOs
- 5.5 Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 South Korea
- 5.5.3.5 Australia
- 5.5.3.6 Rest of Asia
- 5.5.4 Middle East and Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East and Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 Thermo Fisher Scientific
- 6.3.2 Agilent Technologies Inc.
- 6.3.3 Merck KGaA (MilliporeSigma)
- 6.3.4 Danaher Corporation (Beckman Coulter & Cytiva)
- 6.3.5 QIAGEN N.V.
- 6.3.6 PerkinElmer Inc.
- 6.3.7 Illumina Inc.
- 6.3.8 Bio-Rad Laboratories Inc.
- 6.3.9 Tecan Group Ltd.
- 6.3.10 Biotage AB
- 6.3.11 Norgen Biotek Corp.
- 6.3.12 Eppendorf AG
- 6.3.13 Hamilton Company
- 6.3.14 Promega Corporation
- 6.3.15 LGC Biosearch Technologies
- 6.3.16 Takara Bio Inc.
- 6.3.17 Becton, Dickinson and Company
- 6.3.18 Fluidigm Corporation
- 6.3.19 Analytik Jena GmbH
- 6.3.20 Brooks Life Sciences
7 Market Opportunities & Future Outlook
- 7.1 White-Space & Unmet-Need Assessment




