PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1850336

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1850336
Laboratory Filtration - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
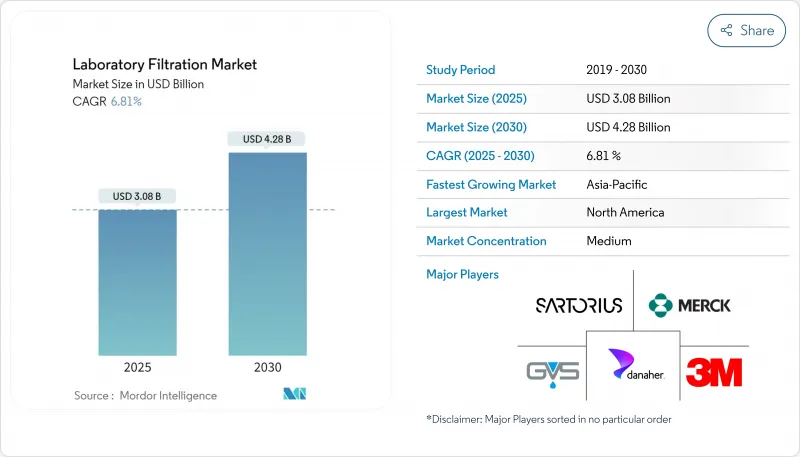
The Laboratory Filtration Market size is estimated at USD 4.70 billion in 2025, and is expected to reach USD 6.91 billion by 2030, at a CAGR of 8.02% during the forecast period (2025-2030).
Rising biopharmaceutical production volumes, rapid adoption of single-use process technologies, and escalating purity requirements in advanced research workflows underpin this expansion. Precision-grade microfiltration continues to anchor routine clarification steps, while breakthrough nanofiltration platforms are gaining traction for molecular-level separations in cell and gene therapy pipelines. Heightened outsourcing to contract research and development manufacturing organizations (CRDMOs) is widening access to flexible filtration assemblies, and sustainability initiatives are accelerating the shift toward PFAS-free membranes. Competitive differentiation now revolves around virus-retentive performance, automation readiness, and digital compatibility, encouraging a steady wave of product upgrades and platform integrations across the laboratory filtration market.
Global Laboratory Filtration Market Trends and Insights
Rapid expansion of biologics manufacturing
Biologics pipelines are scaling quickly in monoclonal antibodies, recombinant proteins, vaccines, and cell-based therapies. Downstream purification now demands sterile, virus-retentive filters that handle higher titers without compromising biomolecule integrity. Asahi Kasei Medical's Planova FG1 filter, released in October 2024, demonstrates a seven-fold increase in volumetric throughput for antibody processing while preserving virus clearance performance. Strong demand for single-use bag-integrated cartridges further propels the laboratory filtration market as manufacturers build flexible plants capable of rapid product changeovers.
Miniaturization of genomics & proteomics workflows
High-throughput sequencing and multiplexed proteomics have condensed sample volumes from milliliters to microliters. Filtration devices compatible with 96- and 384-well plates are now standard in next-generation sequencing (NGS) library preparation and biomarker validation assays. Cytiva's Whatman Mini-UniPrep G2 syringeless filters combine protein precipitation, particulate removal, and autosampler vial integration in one step, cutting plastic use and hands-on time while meeting the precision needs of ultrahigh-performance liquid chromatography (UHPLC). Automation-friendly formats position the laboratory filtration market for sustained uptake in digital, walk-away genomics platforms.
Reuse of sterilizing-grade filters & high cost
Laboratories under fiscal pressure often attempt to recycle expensive sterilizing-grade membranes, reducing consumable spend by up to 50% but amplifying contamination and validation risks. The burden is acute in small academic labs and resource-limited geographies, where filter integrity testing infrastructure may be lacking.
Other drivers and restraints analyzed in the detailed report include:
- Growth of CROs & CDMOs
- Rise in R&D spending by biotechnology industries
- Variability in nanoporous membranes
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
The laboratory filtration market size for microfiltration amounted to 40.2% of global revenue in 2024, underscoring its ubiquity in microorganism removal and sample clarification tasks. Nanofiltration, however, is on pace to compound at 9.6% annually to 2030 as laboratories adopt molecular-level cutoffs for virus clearance, salt-selective separations, and therapeutic-grade buffer production. The FilmTec LiNE-XD element from DuPont illustrates this shift, achieving high lithium passage while excluding multivalent ions critical to battery-material quality control.
Ultrafiltration and reverse osmosis remain cornerstones for protein concentration and ultrapure water generation respectively. Hybrid membranes combining graphene oxide channels with polymer backbones point toward the next wave of cross-disciplinary breakthroughs. Such innovations blur legacy boundaries, compelling vendors to articulate performance metrics in terms relevant to biotherapeutics, semiconductor rinsing, and environmental testing alike.
The Laboratory Filtration Market Report is Segmented by Technology (Microfiltration, Ultrafiltration, Reverse Osmosis, and More), Product (Filtration Media, Filtration Assemblies, and Filtration Accessories), End User (Pharmaceutical and Biotechnology Companies, and More), and Geography (North America, Europe, Asia-Pacific, Middle East and Africa, and South America). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America generated the largest share 36.4% of the laboratory filtration market in 2024 owing to its advanced pharmaceutical R&D, dense biotech clusters, and strict quality regulations. Boston's Kendall Square, the San Francisco Bay Area, and San Diego collectively orchestrate high-throughput biologics discovery pipelines, securing recurring orders for sterilizing-grade media, depth filters, and disposable capsules. Canada's biologics capacity expansion programs and Mexico's cost-competitive fill-finish facilities further elevate regional unit volumes.
Asia-Pacific is the most dynamic arena, advancing at a 10.7% CAGR through 2030. China's provincial life-science parks are outfitting greenfield plants with single-use filtration trains to support mRNA vaccines and gene-edited cell therapies. Singapore's Biomedical Sciences Initiative and South Korea's pharmaceutical stimulus packages intensify local demand for automation-ready filtration units, while Japan sustains premium segments with ultra-high precision membrane grades. India's generics producers reinforce bulk-drug filtration throughput, emphasizing cost-efficient media that maintain compliance with PIC/S harmonization guidelines.
Europe maintains significant weight in the global laboratory filtration market. Germany's engineering heritage fosters steady adoption of advanced membrane modules, and the United Kingdom's cell therapy manufacturing ecosystem drives specialty filter designs optimized for viral vector purification. France, Switzerland, and the Nordic countries extend the region's footprint with strong analytical testing sectors. In South America, Brazil anchors investment in vaccine fill-finish lines, whereas the Middle East & Africa are witnessing incremental gains tied to national immunization and water-quality programs.
- 3M
- Merck
- Danaher
- Sartorius
- Thermo Fisher Scientific
- GVS SpA
- Cole-Parmer Instrument LLC
- Agilent Technologies
- Ahlstrom-Munksjo
- Abcam
- Purolite Corp.
- Repligen Corp.
- Parker Hannifin
- Sterlitech Corp.
- Advantec MFS
- GE Healthcare
- Cobetter Filtration Equipment Co.
- Graver Technologies LLC
- Meissner Filtration Products
- Porvair Filtration Group
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rapid Expansion of Biologics & Cell-Therapy Manufacturing Requiring Sterile Filtration
- 4.2.2 Miniaturization of Genomics & Proteomics Workflows Fueling Microplate-Based Filtration Demand
- 4.2.3 Growth of CROs & CDMOs Boosting Cost-Effective Filtration Assemblies
- 4.2.4 Rise in Research and Development Spending by the Biotechnology Industries
- 4.2.5 Technological Advancements in Laboratory Filtration
- 4.2.6 Sustainability-driven Filtration Innovation
- 4.3 Market Restraints
- 4.3.1 Reuse of Sterilizing-grade Filters and High Cost of Specialized Filters
- 4.3.2 Variability in Nanoporous Membranes Limiting Reproducibility for Biologics Assays
- 4.3.3 Consolidation of Pharma Buyers Increasing Margin Pressure on Filter Vendors
- 4.3.4 PFAS-Driven Reformulation Costs
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitutes
- 4.4.5 Competitive Rivalry
5 Market Size & Growth Forecasts (Value in USD)
- 5.1 By Technology
- 5.1.1 Microfiltration
- 5.1.2 Ultrafiltration
- 5.1.3 Reverse Osmosis
- 5.1.4 Vacuum Filtration
- 5.1.5 Nanofiltration
- 5.2 By Product
- 5.2.1 Filtration Media
- 5.2.1.1 Membrane Filters
- 5.2.1.2 Filter Papers
- 5.2.1.3 Filtration Microplates
- 5.2.1.4 Syringeless Filters
- 5.2.1.5 Syringe Filters
- 5.2.1.6 Capsule Filters
- 5.2.2 Filtration Assemblies
- 5.2.2.1 Microfiltration Assemblies
- 5.2.2.2 Ultrafiltration Assemblies
- 5.2.2.3 Vacuum Filtration Assemblies
- 5.2.2.4 Reverse Osmosis Assemblies
- 5.2.2.5 Nanofiltration Assemblies
- 5.2.3 Filtration Accessories
- 5.2.1 Filtration Media
- 5.3 By End User
- 5.3.1 Pharmaceutical and Biotechnology Companies
- 5.3.2 Academic and Research Institutes
- 5.3.3 CRO and CDMO
- 5.3.4 Hospital and Diagnostic Laboratories
- 5.4 By Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East & Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East & Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Share Analysis
- 6.4 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.4.1 3M
- 6.4.2 Merck KGaA
- 6.4.3 Danaher Corporation
- 6.4.4 Sartorius AG
- 6.4.5 Thermo Fisher Scientific Inc.
- 6.4.6 GVS SpA
- 6.4.7 Cole-Parmer Instrument LLC
- 6.4.8 Agilent Technologies Inc.
- 6.4.9 Ahlstrom-Munksjo
- 6.4.10 Abcam PLC
- 6.4.11 Purolite Corp.
- 6.4.12 Repligen Corp.
- 6.4.13 Parker Hannifin
- 6.4.14 Sterlitech Corp.
- 6.4.15 Advantec MFS Inc.
- 6.4.16 GE Healthcare
- 6.4.17 Cobetter Filtration Equipment Co.
- 6.4.18 Graver Technologies LLC
- 6.4.19 Meissner Filtration Products
- 6.4.20 Porvair Filtration Group
7 Market Opportunities & Future Outlook
- 7.1 White-Space & Unmet-Need Assessment




