PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851171

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851171
Escherichia Coli Testing - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The Escherichia Coli Testing market reached USD 2.40 billion in 2025 and is forecast to reach USD 3.31 billion by 2030, advancing at a 6.62% CAGR.
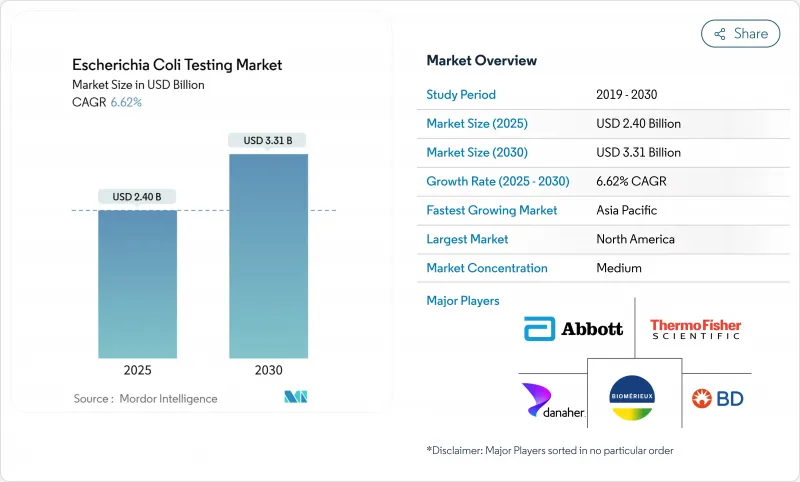
Market expansion benefits from stricter food-borne outbreak surveillance, rising water-quality monitoring, and clinical adoption of rapid molecular diagnostics. Growth also reflects investment in portable biosensor platforms that shorten time-to-result, as well as AI-enhanced culture workflows that automate colony enumeration. While capital requirements for fully automated PCR workstations temper near-term demand among smaller laboratories, continued regulatory tightening and antimicrobial-resistance monitoring offset this restraint. The Escherichia Coli Testing market therefore demonstrates resilient momentum as users transition from culture-based techniques toward integrated molecular and digital solutions.
Global Escherichia Coli Testing Market Trends and Insights
Surging Molecular-PCR Adoption for Multiplex Diarrheal Panels
Healthcare networks are moving from single-pathogen assays to multiplex panels that detect several gastrointestinal organisms in a single test, cutting diagnostic time from days to hours. Platforms such as the FilmArray GI panel provide results for 22 pathogens, including E. coli O157, within 1 hour, allowing physicians to tailor therapy quickly. Artificial intelligence further refines specificity by distinguishing true positives from background noise, and studies show hospital stays fall when rapid PCR replaces culture. The trend supports premium pricing, particularly in emergency departments where rapid answers improve antimicrobial stewardship.
Food-Borne Outbreak Surveillance Mandates
Revisions to the Food Safety Modernization Act, effective July 2024, compel stronger pre-harvest agricultural water testing. High-profile incidents, such as the 2024 fast-food outbreak, triggered adoption of on-site, portable assays so processors verify cleanliness before distribution. Automated systems capable of analysing diverse matrices enable compliance while controlling costs, and AI-driven risk models prioritise sampling based on weather and historical contamination patterns.
Capital Intensity of Fully Automated Stool PCR Work-Cells
Acquiring an end-to-end stool PCR robot costs between USD 500,000 and USD 2 million. Beyond purchase price, facilities must add dedicated space, ventilation, and backup power. Smaller diagnostic centres struggle to justify investment given limited volumes and ongoing maintenance.
Other drivers and restraints analyzed in the detailed report include:
- Growth in Decentralized Water-Quality Testing at Utilities
- Expansion of Livestock AMR Monitoring Programs
- Reagent Stock-Outs in Low-Resource Public Labs
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Escherichia Coli Testing market data show clinical applications held 60.24% share in 2024, and this segment is forecast to advance at 9.24% CAGR through 2030. Polymerase chain reaction remains the workhorse in hospitals because it returns results within hours and detects low bacterial loads. The Escherichia Coli Testing market size for clinical PCR solutions continues to rise as stool and urine diagnostics expand beyond tertiary centres into urgent-care clinics. LAMP kits, which operate at a single temperature, appeal to point-of-care sites where instrumentation budgets are constrained. Enzyme immunoassays still serve cost-sensitive programs, but their share erodes as molecular platforms become cheaper per test.
Environmental assays, spanning water, soil, and wastewater matrices, rely on membrane filtration for regulatory reporting, yet demand for automated enzyme-substrate methods grows where rapid decisions are required. Chromogenic culture media now visualise suspect colonies within 24 hours, trimming confirmation cycles. Rapid biosensor-based tests hold the highest incremental growth because they combine nanostructured surfaces with microfluidics, delivering results in minutes for on-site responders. This shift positions the Escherichia Coli Testing market to embrace hybrid workflows that blend culture validation with molecular speed.
Escherichia Coli Testing Market Report is Segmented by Test (Clinical Testing [Polymerase Chain Reaction, Enzyme Immunoassays], and Environmental Testing [Membrane Filtration, and More]), Sample Type (Water, Food & Beverage and More), End User (Hospitals & Clinics, Diagnostic Laboratories and More) and Geography (North America, Europe, Asia-Pacific and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America held 37.63% of Escherichia Coli Testing market revenue in 2024 owing to robust EPA and FDA mandates that require routine testing across drinking water and produce supply chains. AI-ready culture imagers and fully automated PCR work-cells see strong uptake in the United States as laboratories chase productivity gains and tighter turnaround targets. The 2024 FDA Laboratory Developed Tests rule raises the compliance bar, favouring companies with established quality systems.
Asia Pacific posts the highest 9.04% regional CAGR through 2030, driven by rapid urbanisation and investments in water infrastructure. Low-cost membrane filtration kits and smartphone-enabled biosensors close testing gaps in peri-urban areas. Governments in India and Southeast Asia add water testing to smart-city programs, while China's five-year plan prioritises safe food supply chains, boosting demand for high throughput immunoassay lines. Regional reagent manufacturing lowers freight and cold-chain costs, further fuelling Escherichia Coli Testing market growth.
Europe maintains steady contribution, supported by stringent food and water directives and a mature network of accredited laboratories. The region's focus on sustainability drives adoption of energy-positive water treatment combined with continuous microbial monitoring. Collaborative projects like the bioMerieux-Illumina agreement illustrate Europe's leadership in next-generation sequencing surveillance, merging epidemiological insight with routine testing. The Middle East and Africa represent emerging frontiers where infrastructure investment and donor-funded public-health programs underpin incremental market demand, particularly for portable kits capable of withstanding high ambient temperatures and intermittent power.
- Abbott Laboratories
- Beckton Dickinson
- Bio-Rad Laboratories
- Danaher (Cepheid & Beckman Coulter)
- Thermo Fisher Scientific
- Eurofins
- IDEXX
- Merck KGaA (MilliporeSigma)
- Roche
- HiMedia Laboratories
- Hach (Danaher subsidiary)
- rqmicro AG
- Hygiena LLC
- NEMIS Technologies
- Certest Biotec
- Fujifilm Wako Pure Chemical
- Eiken Chemical
- Microbiologics
- Hardy Diagnostics
- Lonza Group
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Surging Molecular-PCR Adoption For Multiplex Diarrheal Panels
- 4.2.2 Food-Borne Outbreak Surveillance Mandates
- 4.2.3 Growth In Decentralized Water-Quality Testing At Utilities
- 4.2.4 Expansion Of Livestock AMR Monitoring Programs
- 4.2.5 Portable Biosensor-On-Chip Innovations For On-Site Screening
- 4.2.6 AI-Augmented Image Analytics In Culture Media Reading
- 4.3 Market Restraints
- 4.3.1 Capital Intensity Of Fully Automated Stool PCR Work-Cells
- 4.3.2 Reagent Stock-Outs In Low-Resource Public Labs
- 4.3.3 Regulatory Lag For Validating Next-Gen Microfluidic Kits
- 4.3.4 False-Positive Inflation From Sample Matrix Inhibitors
- 4.4 Value / Supply-Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technology Outlook
- 4.7 Porter's Five Forces Analysis
- 4.7.1 Bargaining Power of Suppliers
- 4.7.2 Bargaining Power of Buyers
- 4.7.3 Threat of New Entrants
- 4.7.4 Threat of Substitutes
- 4.7.5 Intensity of Competitive Rivalry
5 Market Size and Growth Forecasts (Value-USD)
- 5.1 By Test
- 5.1.1 Clinical Testing
- 5.1.1.1 Polymerase Chain Reaction (PCR)
- 5.1.1.2 Loop-mediated Isothermal Amplification (LAMP)
- 5.1.1.3 Enzyme Immunoassays (EIA/ELISA)
- 5.1.1.4 Chromogenic Culture Media
- 5.1.1.5 Rapid Biosensor-based Assays
- 5.1.2 Environmental Testing
- 5.1.2.1 Membrane Filtration
- 5.1.2.2 Multiple Tube Fermentation
- 5.1.2.3 Enzyme Substrate Method
- 5.1.1 Clinical Testing
- 5.2 By Sample Type
- 5.2.1 Water
- 5.2.2 Food & Beverage
- 5.2.3 Clinical Stool / Urine
- 5.2.4 Animal Feed & Livestock
- 5.3 By End User
- 5.3.1 Hospitals & Clinics
- 5.3.2 Diagnostic Laboratories
- 5.3.3 Water Utilities & Waste-Water Treatment Plants
- 5.3.4 Food Processing Companies
- 5.3.5 Government & Public-Health Agencies
- 5.4 By Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products and Services, and Recent Developments)
- 6.3.1 Abbott Laboratories
- 6.3.2 Becton, Dickinson & Company
- 6.3.3 Bio-Rad Laboratories
- 6.3.4 Danaher (Cepheid & Beckman Coulter)
- 6.3.5 Thermo Fisher Scientific
- 6.3.6 Eurofins Scientific
- 6.3.7 IDEXX Laboratories
- 6.3.8 Merck KGaA (MilliporeSigma)
- 6.3.9 Roche Diagnostics
- 6.3.10 HiMedia Laboratories
- 6.3.11 Hach (Danaher subsidiary)
- 6.3.12 rqmicro AG
- 6.3.13 Hygiena LLC
- 6.3.14 NEMIS Technologies
- 6.3.15 Certest Biotec
- 6.3.16 Fujifilm Wako Pure Chemical
- 6.3.17 Eiken Chemical
- 6.3.18 Microbiologics
- 6.3.19 Hardy Diagnostics
- 6.3.20 Lonza Group
7 Market Opportunities and Future Outlook
- 7.1 White-Space and Unmet-Need Assessment




