PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851342

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851342
China Cardiovascular Devices - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The China cardiovascular devices market size stands at USD 3.16 billion in 2025 and is forecast to reach USD 4.19 billion by 2030, advancing at a 5.79% CAGR over the period.
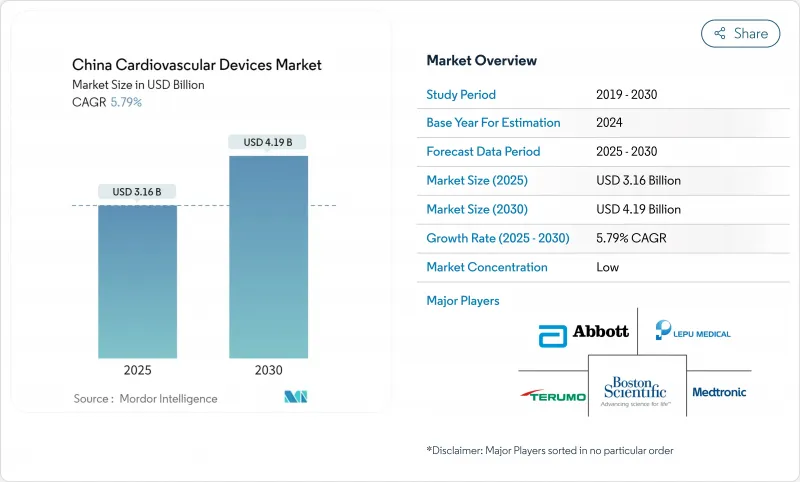
Rising life expectancy, an estimated 330 million cardiovascular patients, and the policy tilt of "Healthy China 2030" continue to lift procedure volumes and the uptake of both interventional and monitoring technologies . Price compression sparked by volume-based procurement (VBP) on coronary stents triggered a 95% price fall yet lifted overall stent usage by nearly 10%, illustrating how cost reform can amplify device penetration. Domestic producers are capitalising on these reforms, aided by "Made in China 2025," which targets 70% local production of mid- to high-end cardiovascular equipment by 2025 . Simultaneously, China's expanding chest-pain-center network has cut median door-to-balloon times from 117.7 minutes to 46.9 minutes, adding momentum to advanced imaging, guidewire, and emergency monitoring demand.
China Cardiovascular Devices Market Trends and Insights
Rapid expansion of China's chest-pain centers accrediting advanced interventional device adoption
The national roll-out of chest-pain centers has shortened door-to-balloon intervals to as low as 46.9 minutes, driving stronger uptake of high-performance guidewires, drug-eluting stents, and intravascular imaging systems . Integration of artificial-intelligence triage further lifted primary PCI rates within 90 minutes from 24.47% to 60.41% and pushed connected ECG platforms that cut 30-day mortality from 4.14% to 2.73% . National standards maintained by the Chinese Cardiovascular Association make performance metrics public, prompting county-level hospitals to standardise product choices toward devices demonstrating clinical efficiency. This standardisation increases procurement predictability and compresses time-to-tender for domestic innovators aligned with evidence-based protocols. As the program expands into central and western provinces, device makers that couple competitive pricing with proven clinical outcomes are positioned to capture incremental volumes within the China cardiovascular devices market.
Government "Made in China 2025" initiatives boosting domestic cardiovascular device innovation
The industrial policy goal of 70% domestic supply for mid- to high-end cardiovascular equipment has redirected capital into research pipelines, with leading local firms dedicating 11-14% of sales to R&D, well above the global med-tech average. Resulting product launches span coronary bifurcation stents, pulsed-field ablation systems, and magnetic levitation pumps, all cleared through the National Medical Products Administration's (NMPA) innovation channel. While foreign brands still dominate complex heart-valve segments, Chinese entrants now match global peers in many delivery-system and polymer-coating technologies, shifting procurement in provincial tenders toward locally registered SKUs. Industry stakeholders expect the localisation push to permeate peripheral vascular and electrophysiology niches by 2027, reinforcing the structural tilt of the China cardiovascular devices market toward home-grown solutions.
Price erosion due to ongoing VBP rounds on high-value consumables
Successive VBP extensions from stents to pacemakers, defibrillators, and valves have saved CNY 260 billion across three years but crimped average selling prices and gross profit margins for premium lines. Multinationals face tougher trade-offs between margin retention and share maintenance, while domestic firms rely on scale economics to protect bottom lines. Although higher volumes cushion revenue, the short-run impact is negative for top-line growth, moderating the overall China cardiovascular devices market CAGR.
Other drivers and restraints analyzed in the detailed report include:
- Rising prevalence of atrial fibrillation in an aging population elevating demand for cardiac rhythm management devices
- Accelerated conditional approval pathway for breakthrough cardiovascular implants by NMPA
- Capacity bottlenecks in qualified cath labs outside top-tier cities
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Therapeutic and Surgical Devices contributed 68.20% of the China cardiovascular devices market in 2024, buoyed by 2,200 annual coronary interventions at leading centers and VBP-induced volume surges. The cardiac-rhythm-management niche accelerated after NMPA cleared MicroPort Sorin's new pacing lead in February 2025, underscoring local progress in hermetic sealing and lead-coil metallurgy. Intravascular lithotripsy, renal denervation, and next-gen drug-coated balloons form the emerging wave, with localisation tipping cost-of-goods lower than imports. Over the forecast period, domestic innovators are expected to keep displacing foreign incumbents across mid-complexity implants, reinforcing the primacy of this category within the China cardiovascular devices market.
Diagnostic and Monitoring Devices are projected to expand at a 6.98% CAGR through 2030. AI-enabled ECG analytics integrated into smart wearables bring early detection into homes, while hospital chest-pain networks still buy high-throughput CT and MRI scanners for triage optimisation. Cardiac-biomarker assays backed by local reagent makers support rapid rule-out protocols, serving the 330 million strong patient pool . Cloud-native data platforms align with the NMPA's revised cybersecurity guidance, helping vendors secure faster approval for software-as-medical-device modules. Given rising chronic-disease management at home, connected diagnostics should keep edging wallet share away from purely interventional SKUs, adding depth to the China cardiovascular devices market size.
The China Cardiovascular Devices Market Report is Segmented by Device Type (Diagnostic & Monitoring Devices, Therapeutic & Surgical Devices), Application (Coronary Artery Disease, Arrhythmia & Conduction Disorders, Heart Failure & Cardiomyopathy, and More), End-User (Hospitals & Cardiac Centres, Ambulatory Surgical Centres, and More). The Market Forecasts are Provided in Terms of Value (USD).
List of Companies Covered in this Report:
- MicroPort
- Lepu Medical Technology (Beijing) Co., Ltd.
- Abbott Laboratories
- Medtronic
- Boston Scientific
- Edward Lifesciences
- Terumo
- Mindray
- Shanghai MicroPort Endovascular MedTech (EMT)
- BIOTRONIK
- Jiangsu Yuyue Medical Equipment & Supply Co., Ltd.
- Sino Medical Sciences Technology Inc. (SMST)
- JW Medical Systems Ltd.
- Peijia Medical Limited
- Venus Medtech
- Kewei Medical Instrument Co., Ltd.
- Jiangsu Yuyue Medical Instruments Co., Ltd.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rapid Expansion of China's Chest-Pain Centers Accrediting Advanced Interventional Device Adoption
- 4.2.2 Government "Made in China 2025" Initiatives Boosting Domestic Cardiovascular Device Innovation
- 4.2.3 Rising Prevalence of Atrial Fibrillation in Aging Chinese Population Elevating Demand for CRM Devices
- 4.2.4 National Volume-Based Procurement (VBP) for Drug-Eluting Stents Triggering High-Volume Domestic Production
- 4.2.5 Accelerated Conditional Approval Pathway for Breakthrough Cardiovascular Implants by NMPA
- 4.3 Market Restraints
- 4.3.1 Price Erosion due to Ongoing VBP Rounds on High-Value Consumables
- 4.3.2 Capacity Bottlenecks in Qualified Cath Labs Outside Top-Tier Cities
- 4.3.3 Clinical Talent Shortage for TAVR and Left Atrial Appendage Closure Procedures
- 4.3.4 Heightened Post-Market Surveillance Scrutiny on Imported Implantables
- 4.4 Supply-Chain Analysis
- 4.5 Regulatory Outlook
- 4.6 Technological Outlook
- 4.7 Porter's Five Forces
- 4.7.1 Threat of New Entrants
- 4.7.2 Bargaining Power of Suppliers
- 4.7.3 Bargaining Power of Buyers
- 4.7.4 Threat of Substitutes
- 4.7.5 Industry Rivalry
- 4.8 Pricing Analysis
5 Market Size & Growth Forecasts (Value, USD Billion)
- 5.1 By Device Type
- 5.1.1 By Product Type
- 5.1.1.1 Diagnostic & Monitoring Devices
- 5.1.1.1.1 ECG Systems
- 5.1.1.1.2 Remote Cardiac Monitor
- 5.1.1.1.3 Cardiac MRI
- 5.1.1.1.4 Cardiac CT
- 5.1.1.1.5 Echocardiography / Ultrasound
- 5.1.1.1.6 Fractional Flow Reserve (FFR) Systems
- 5.1.1.2 Therapeutic & Surgical Devices
- 5.1.1.2.1 Coronary Stents
- 5.1.1.2.1.1 Drug-Eluting Stents
- 5.1.1.2.1.2 Bare-Metal Stents
- 5.1.1.2.1.3 Bioresorbable Stents
- 5.1.1.2.2 Catheters
- 5.1.1.2.2.1 PTCA Balloon Catheters
- 5.1.1.2.2.2 IVUS/OCT Catheters
- 5.1.1.2.3 Cardiac Rhythm Management
- 5.1.1.2.3.1 Pacemakers
- 5.1.1.2.3.2 Implantable Cardioverter Defibrillators
- 5.1.1.2.3.3 Cardiac Resynchronization Therapy Devices
- 5.1.1.2.4 Heart Valves
- 5.1.1.2.4.1 TAVR/TAVI
- 5.1.1.2.4.2 Mechanical Valves
- 5.1.1.2.4.3 Tissue/Bioprosthetic Valves
- 5.1.1.2.5 Ventricular Assist Devices
- 5.1.1.2.6 Artificial Hearts
- 5.1.1.2.7 Grafts & Patches
- 5.1.1.2.8 Other Cardiovascular Surgical Devices
- 5.1.2 By Application
- 5.1.2.1 Coronary Artery Disease
- 5.1.2.2 Arrhythmia & Conduction Disorders
- 5.1.2.3 Heart Failure & Cardiomyopathy
- 5.1.2.4 Structural & Congenital Heart Defects
- 5.1.2.5 Peripheral Vascular Disease
- 5.1.3 By End User
- 5.1.3.1 Hospitals & Cardiac Centres
- 5.1.3.2 Ambulatory Surgical Centres
- 5.1.3.3 Cardiology/EP Clinics
- 5.1.3.4 Home-care & Remote Monitoring Programs
- 5.1.1 By Product Type
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 MicroPort Scientific Corporation
- 6.3.2 Lepu Medical Technology (Beijing) Co., Ltd.
- 6.3.3 Abbott Laboratories
- 6.3.4 Medtronic plc
- 6.3.5 Boston Scientific Corporation
- 6.3.6 Edwards Lifesciences Corporation
- 6.3.7 Terumo Corporation
- 6.3.8 Shenzhen Mindray Bio-Medical Electronics Co., Ltd.
- 6.3.9 Shanghai MicroPort Endovascular MedTech (EMT)
- 6.3.10 Biotronik SE & Co. KG
- 6.3.11 Jiangsu Yuyue Medical Equipment & Supply Co., Ltd.
- 6.3.12 Sino Medical Sciences Technology Inc. (SMST)
- 6.3.13 JW Medical Systems Ltd.
- 6.3.14 Peijia Medical Limited
- 6.3.15 Venus Medtech (Hangzhou) Inc.
- 6.3.16 Kewei Medical Instrument Co., Ltd.
- 6.3.17 Jiangsu Yuyue Medical Instruments Co., Ltd.
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-Need Assessment




