PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851804

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851804
Biomarkers - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The biomarkers market stood at USD 57.27 billion in 2025 and is forecast to reach USD 97.42 billion by 2030, delivering an 11.21% CAGR.
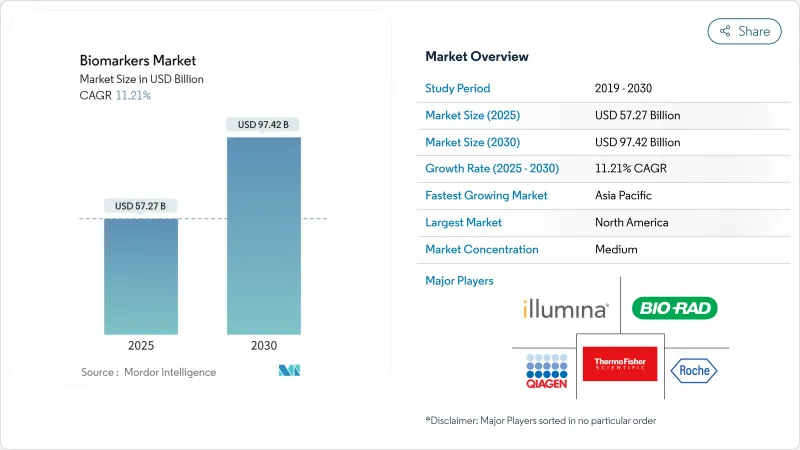
Momentum reflects artificial-intelligence-enabled discovery pipelines, wider regulatory acceptance of digital endpoints, and the push for precision medicine across oncology, immunology, neurology, and cardiology. Continued breakthrough-device designations, expanding multi-omics toolkits, and reimbursement pathways that reward targeted therapies fuel uptake of validated tests in routine care. Companion diagnostics now anchor treatment decisions, especially in oncology, where liquid biopsy and DNA methylation assays widen access to early detection and therapy matching. Investments in proteomics platforms, cloud bioinformatics, and real-world evidence solutions position vendors to capture recurring revenue from consumables, services, and software. Yet complex reimbursement policies and data-privacy regulations temper near-term adoption curves.
Global Biomarkers Market Trends and Insights
Rising Prevalence of Life-Threatening Diseases
Chronic and life-threatening diseases are accelerating demand for refined biomarker panels. Rheumatoid arthritis registers 200,000 new U.S. cases each year, spurring adoption of 14-3-3 η protein assays that reach 74% sensitivity and 90% specificity for early detection. Age-related disorders amplify the need for minimally invasive screens; for Alzheimer's, blood-based pTau217 recently obtained FDA breakthrough designation and improves accessibility beyond cerebrospinal fluid testing . Healthcare systems pivot toward preventive medicine, prompting multiplex platforms capable of measuring markers across oncology, cardiology, and neurodegeneration in a single run. Such breadth keeps the biomarkers market on a firm growth path.
Growing Demand for Early & Accurate Diagnosis
Early intervention strategies make biomarkers pivotal predictive instruments rather than confirmatory tools. CLAIRITY BREAST, an AI model that forecasts five-year breast-cancer risk from mammograms, illustrates how imaging biomarkers support proactive screening. Liquid biopsy assays detecting circulating tumor-DNA methylation now achieve 96.67% sensitivity for hepatocellular carcinoma within a 24-hour workflow . Wearables extend detection beyond clinics, with continuous monitoring of cardiometabolic indicators that trigger timely physician alerts. Artificial-intelligence-augmented analytics shorten interpretation time, easing integration into varied care settings and expanding the biomarkers market.
Complex Reimbursement & Regulatory Pathways
Divergent global rules slow commercialization. The FDA will phase out laboratory-developed-test enforcement discretion over four years, imposing device-level controls on clinical labs. Europe's In Vitro Diagnostic Regulation tightened evidence demands, while Asia-Pacific requirements remain inconsistent. Payers often require extra real-world evidence, delaying revenue for innovators and marginally cooling biomarkers market expansion.
Other drivers and restraints analyzed in the detailed report include:
- Advancements in Multi-Omics Technologies
- Expansion of Companion Diagnostics in Oncology
- High Assay Development & Validation Costs
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
The cancer segment generated 46.52% of 2024 revenue within the biomarkers market. Decades of investment in genetic and protein markers, combined with fast-track companion diagnostic approvals, sustain leadership. Epigenetic liquid biopsy tests now identify multiple tumor types from a 10 ml blood draw, improving access to early treatment.
Immunological disorders are advancing at an 11.98% CAGR toward 2030, narrowing the gap. Rising autoimmune prevalence and the validation of markers such as 14-3-3 η protein in seronegative rheumatoid arthritis open new clinical workflows. Broader multiplex panels capable of tracking cytokine storms and treatment response fuel long-term growth in this slice of the biomarkers market.
Efficacy biomarkers accounted for 58.23% of 2024 spending, reflecting their use in predicting progression, guiding therapy choice, and serving as surrogate endpoints. Oncology and cardiology trials rely on established markers to cut timelines and optimize dosing.
Safety biomarkers climb fastest at 11.81% CAGR as regulators require human-relevant toxicity indicators before market authorization. AI-enabled in-vitro models read renal and hepatic stress signals within hours, supporting earlier attrition decisions. Expansion of these panels underpins risk-mitigation strategies and enlarges the biomarkers market size for pharmacovigilance.
The Biomarkers Market is Segmented by Disease (Cancer, Cardiovascular Disorders, and More), Type (Efficacy Biomarkers, Safety Biomarkers, and More), Mechanism (Genetic Biomarkers, Epigenetic Biomarkers, and More), Application (Clinical Diagnostics and More), Product (Consumables, Instruments, and More) and Geography (North America, Europe, Asia-Pacific, and More). The Market and Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America held 42.88% of global revenue in 2024 owing to FDA programs that qualify biomarkers for regulatory submissions and streamline payer coverage. Robust reimbursement and an extensive hospital network maintain high test volumes. Academic-industry consortia fast-track translation from bench to bedside, consolidating regional influence in the biomarkers market.
Asia Pacific is advancing at an 11.89% CAGR through 2030. China's 24-measure regulatory overhaul hastens innovative-device clearance, while Japan's national biotechnology plan targets 15 trillion yen market output by decade end. Aging demographics and new reimbursement codes for liquid biopsy spur rapid adoption, positioning Asia Pacific as a prime demand center for the biomarkers market.
Europe maintains consistent growth under the In Vitro Diagnostic Regulation's stringent evidence demands. Cross-border research networks produce large cohorts that validate digital and multi-omics biomarkers for chronic disease management. Middle East, Africa, and South America offer greenfield opportunities as health-system modernization and medical tourism stimulate demand for advanced diagnostics within an emerging biomarkers market ecosystem.
- Abbott Laboratories
- Roche
- Thermo Fisher Scientific
- QIAGEN
- Danaher Corp. ( Beckman Coulter )
- Siemens Healthineers
- Bio-Rad Laboratories
- Agilent Technologies
- Illumina
- PerkinElmer
- Merck
- Becton Dickinson & Co.
- Hologic
- Sysmex Corp.
- Myriad Genetics
- Epigenomics
- Quanterix Corp.
- GE Healthcare
- bioMerieux
- Atlas Genetics Ltd
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rising Prevalence of Life-Threatening Diseases
- 4.2.2 Growing Demand for Early & Accurate Diagnosis
- 4.2.3 Advancements In Multi-Omics Technologies
- 4.2.4 Expansion Of Companion Diagnostics in Oncology
- 4.2.5 AI-Powered Multi-Modal Biomarker Discovery
- 4.2.6 Proliferation Of Digital Biomarkers
- 4.3 Market Restraints
- 4.3.1 Complex Reimbursement & Regulatory Pathways
- 4.3.2 High Assay Development & Validation Costs
- 4.3.3 Data-Privacy Challenges with Real-World Digital Biomarkers
- 4.3.4 Sample-To-Answer Workflow Variability in Low-Resource Labs
- 4.4 Regulatory Landscape
- 4.5 Technological Outlook
- 4.6 Porters Five Forces Analysis
- 4.6.1 Bargaining Power of Suppliers
- 4.6.2 Bargaining Power of Buyers
- 4.6.3 Threat of New Entrants
- 4.6.4 Threat of Substitutes
- 4.6.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Disease
- 5.1.1 Cancer
- 5.1.2 Cardiovascular Disorders
- 5.1.3 Neurological Disorders
- 5.1.4 Immunological Disorders
- 5.1.5 Renal Disorders
- 5.1.6 Other Diseases
- 5.2 By Type
- 5.2.1 Efficacy Biomarkers
- 5.2.1.1 Prognostic Biomarkers
- 5.2.1.2 Predictive Biomarkers
- 5.2.1.3 Pharmacodynamic Biomarkers
- 5.2.1.4 Surrogate Endpoint Markers
- 5.2.2 Safety Biomarkers
- 5.2.3 Validation Biomarkers
- 5.2.1 Efficacy Biomarkers
- 5.3 By Mechanism
- 5.3.1 Genetic Biomarkers
- 5.3.2 Epigenetic Biomarkers
- 5.3.3 Proteomic Biomarkers
- 5.3.4 Lipidomic Biomarkers
- 5.3.5 Others
- 5.4 By Application
- 5.4.1 Clinical Diagnostics
- 5.4.2 Drug Discovery and Development
- 5.4.3 Personalised Medicine
- 5.4.4 Disease Risk Assessment
- 5.4.5 Others
- 5.5 By Product
- 5.5.1 Consumables
- 5.5.2 Instruments
- 5.5.3 Services and Software
- 5.6 By Geography
- 5.6.1 North America
- 5.6.1.1 United States
- 5.6.1.2 Canada
- 5.6.1.3 Mexico
- 5.6.2 Europe
- 5.6.2.1 Germany
- 5.6.2.2 United Kingdom
- 5.6.2.3 France
- 5.6.2.4 Italy
- 5.6.2.5 Spain
- 5.6.2.6 Rest of Europe
- 5.6.3 Asia-Pacific
- 5.6.3.1 China
- 5.6.3.2 Japan
- 5.6.3.3 India
- 5.6.3.4 Australia
- 5.6.3.5 South Korea
- 5.6.3.6 Rest of Asia-Pacific
- 5.6.4 Middle East and Africa
- 5.6.4.1 GCC
- 5.6.4.2 South Africa
- 5.6.4.3 Rest of Middle East and Africa
- 5.6.5 South America
- 5.6.5.1 Brazil
- 5.6.5.2 Argentina
- 5.6.5.3 Rest of South America
- 5.6.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials, Strategic Info, Market Rank/Share, Products & Services, Recent Developments)
- 6.3.1 Abbott Laboratories
- 6.3.2 F. Hoffmann-La Roche Ltd
- 6.3.3 Thermo Fisher Scientific Inc.
- 6.3.4 Qiagen N.V.
- 6.3.5 Danaher Corp. ( Beckman Coulter )
- 6.3.6 Siemens Healthineers AG
- 6.3.7 Bio-Rad Laboratories Inc.
- 6.3.8 Agilent Technologies Inc.
- 6.3.9 Illumina Inc.
- 6.3.10 PerkinElmer Inc.
- 6.3.11 Merck KGaA
- 6.3.12 Becton Dickinson & Co.
- 6.3.13 Hologic Inc.
- 6.3.14 Sysmex Corp.
- 6.3.15 Myriad Genetics Inc.
- 6.3.16 Epigenomics AG
- 6.3.17 Quanterix Corp.
- 6.3.18 GE Healthcare
- 6.3.19 BioMerieux SA
- 6.3.20 Atlas Genetics Ltd
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-Need Assessment




