PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851905

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851905
Specimen Validity Testing - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The specimen validity testing market size is valued at USD 2.34 billion in 2025 and is projected to reach USD 3.21 billion by 2030, advancing at a 6.53% CAGR.
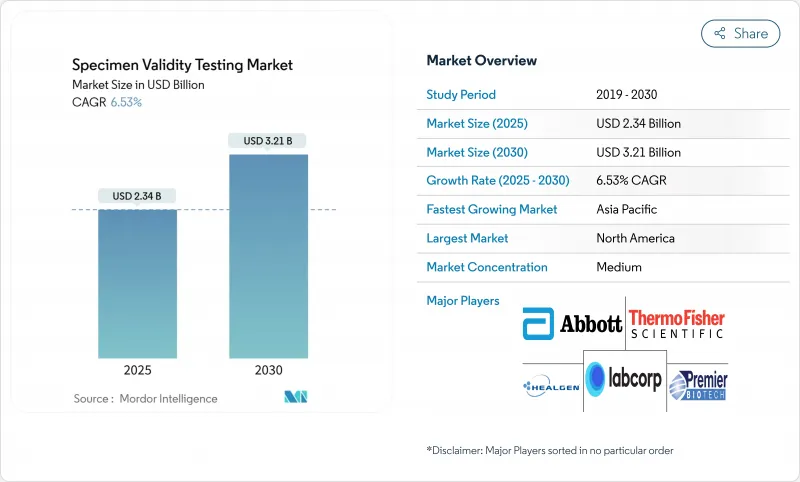
The trajectory reflects tightening workplace-safety mandates, accelerated adoption of point-of-care (POC) testing platforms, and technology upgrades that detect sophisticated adulteration attempts. Employers in transportation, energy, and healthcare continue to widen testing coverage, while government updates-such as the January 2025 federal panel expansion that now includes fentanyl and norfentanyl-raise the integrity bar for all laboratories. Rising telehealth use has also opened a new remote-collection channel, prompting demand for tamper-evident devices and AI-driven analytics that verify specimen integrity in real time. Although non-urine matrices are gaining favor, the specimen validity testing market remains buoyed by high-volume urine programs that still dominate regulated settings.
Global Specimen Validity Testing Market Trends and Insights
Growing Workplace Drug-Testing Mandates
Regulators on six continents are strengthening corporate safety rules, pushing employers to verify not only drug presence but the authenticity of each sample. OSHA-aligned directives in the United States, Canada's Transportation of Dangerous Goods Regulations, and the Oman Ministry of Manpower's compulsory oil-and-gas testing order underscore a collective move toward stricter specimen validity checks. Large conglomerates increasingly embed chain-of-custody software and Medical Review Officer (MRO) oversight into standard operating procedures, mitigating litigation risk after accidents. The resulting demand multiplier lifts recurring sales of temperature strips, oxidant assays, and digital custody systems .
Rapid Adoption of POC SVT Devices
Newly cleared handheld analyzers now complete adulterant screens in under six minutes, meeting the "immediacy" standard required in post-incident investigations. U.S. Food and Drug Administration (FDA) clearance for on-site fentanyl assays has set a precedent for integrating validity checks into single-step POC cups. Employers value reduced downtime, while laboratories see fewer rejected samples, shortening billing cycles. Vendors respond with modular readers that print compliance-ready reports, reinforcing recurring consumable revenues.
Limited Awareness Among Small & Mid-Size Clinics
Smaller healthcare providers often underestimate liability tied to invalid samples. Surveys highlight that 59% of point-of-care implementation barriers relate to training and quality assurance, leaving many clinics without temperature verification or specific-gravity checks. Reimbursement policies now deny claims lacking validity documentation, but knowledge gaps persist, slowing uptake .
Other drivers and restraints analyzed in the detailed report include:
- Telehealth Expansion Spurring At-Home SVT Kits
- Machine-Learning Algorithms for Live Adulteration Detection
- Shift Toward Non-Urine Matrices Reducing SVT Demand
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Products anchored 66.67% of 2024 revenue, sustained by the consumable nature of assay kits, oxidant reagents, creatinine standards, and single-use collection cups. Temperature-indicator strips alone generated a high-velocity replacement cycle, especially in large logistics and energy enterprises. Services, however, post a 7.21% CAGR through 2030 as employers outsource result interpretation and compliance audits to MRO consultants and specialty laboratories. Medical-legal environments favor bundled offerings that merge chain-of-custody management with machine-learning analytics. The specimen validity testing market size for services is forecast to reach USD 1.24 billion by 2030, maintaining momentum despite the dominance of consumables.
The services wave also rides on remote-collection medicine. Telehealth platforms contract third-party labs to verify sample temperature and oxidant levels on arrival, paying premium fees for same-day adjudication. Demand spikes for virtual MRO reviews in cross-border employment programs, broadening addressable revenue and solidifying the specimen validity testing market as a holistic solution rather than a product vertical alone.
The Specimen Validity Testing Market Report is Segmented by Product & Service (Products [Assay Kits, and More], Services), Type (Laboratory Testing, Rapid and POC Testing), End-User (Workplaces, Drug-Screening Laboratories, Pain-Management Centers, and More), and Geography (North America, Europe, Asia-Pacific, Middle East and Africa, South America). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America retained 41.34% revenue in 2024 and remains regulatory pacesetter for the specimen validity testing market. The Department of Health and Human Services requires each regulated urine specimen to document temperature (32-38 °C), pH (4.5-8.0), specific gravity (>=1.003), and creatinine (>=20 mg/dL) immediately after collection SAMHSA. The January 2025 panel revision, which added fentanyl and norfentanyl, further heightened laboratories' need for precise validity confirmation to avoid false negatives under new analyte cutoffs Federal Register. Parallel Department of Transportation mandates apply to roughly 10 million safety-sensitive workers, guaranteeing high test volumes. Canada's privacy-oriented framework reduces random testing but emphasises oral-fluid programs that still rely on temperature and chain-of-custody documentation for defensibility.
Asia-Pacific posts the fastest 7.41% CAGR through 2030, propelled by multinational factories in China and India that embed U.S. Occupational Safety and Health Administration (OSHA) best practices into local operations. Global diagnostic giants have opened regional hubs to supply assay kits and provide MRO services in native languages. Cross-border trade compliance drives companies to adopt U.S.-style chain-of-custody systems, giving the specimen validity testing market fresh scale. Regional governments are studying mandatory drug-testing statutes, particularly in transportation and mining, which would further embed specimen integrity checks.
Europe offers fragmented regulation. German rail operators apply stringent specimen-validity rules modelled on U.S. federal guidelines, whereas France limits testing to safety-sensitive roles and imposes stricter privacy filters. The EU's In Vitro Diagnostic Regulation (IVDR) imposes performance and post-market surveillance obligations on validity devices entering the bloc, raising entry barriers yet standardising quality. Pan-European employers thus lean toward CE-marked collection systems that integrate barcoded integrity seals and cloud custody logs.
The Middle East and Africa follow international oil-and-gas contracting standards. GCC aviation regulators require MRO-overseen validity checks, with chain-of-custody protocols mirroring U.S. SAMHSA guidelines. South Africa's mining houses partner with global laboratories to meet buyer expectations for high-integrity testing metrics. South America is led by Brazil, where Law 13.103/15 compels professional drivers to undergo periodic toxicological assessments that include specimen validity documentation; hair testing's popularity nonetheless demands cross-validation protocols to confirm authenticity. Argentina's expanding automotive sector imports U.S. DOT guidelines to satisfy supply-chain partners.
- Abbott Laboratories
- Thermo Fisher Scientific
- Laboratory Corporation of America (LabCorp)
- Quest Diagnostics
- Siemens Healthineers
- Dragerwerk
- Omega Laboratories
- Premier Biotech Inc.
- Sciteck Inc.
- Alfa Scientific Designs
- QIAGEN
- PerkinElmer
- Orasure Technologies
- Neogen
- Roche
- Healgen Scientific LLC
- Vision Diagnostics Inc.
- Rochester Regional Health (ACM Global Labs)
- Nona Scientific Laboratory
- Applied BioCode Corp.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Workplace Drug-Testing Mandates
- 4.2.2 Rapid Adoption of POC SVT Devices
- 4.2.3 Expansion Of Telehealth Spurring At-Home SVT Kits
- 4.2.4 Machine-Learning Algorithms for Live Adulteration Detection
- 4.2.5 Cannabis Legalization Driving Differentiated SVT Panels
- 4.2.6 Supply-Chain Security Boosting Sterile Sample-Integrity Controls
- 4.3 Market Restraints
- 4.3.1 Limited Awareness Among Small & Mid-Size Clinics
- 4.3.2 Shift Toward Non-Urine Matrices Reducing SVT Demand
- 4.3.3 Data-Privacy Constraints on Biometric SVT Platforms
- 4.3.4 Sporadic Reagent Shortages & Raw-Material Price Spikes
- 4.4 Regulatory Landscape
- 4.5 Porters Five Forces Analysis
- 4.5.1 Threat of New Entrants
- 4.5.2 Bargaining Power of Buyers/Consumers
- 4.5.3 Bargaining Power of Suppliers
- 4.5.4 Threat of Substitute Products
- 4.5.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Product and Service
- 5.1.1 Products
- 5.1.1.1 Assay Kits
- 5.1.1.2 Reagents & Calibrators
- 5.1.1.3 Disposables
- 5.1.2 Services
- 5.1.1 Products
- 5.2 By Type
- 5.2.1 Laboratory Testing
- 5.2.2 Rapid and POC Testing
- 5.3 By End-User
- 5.3.1 Workplaces
- 5.3.2 Law-Enforcement and Criminal-Justice Systems
- 5.3.3 Drug-Screening Laboratories
- 5.3.4 Pain-Management Centers
- 5.3.5 Others
- 5.4 By Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 Abbott
- 6.3.2 Thermo Fisher Scientific
- 6.3.3 Laboratory Corporation of America (LabCorp)
- 6.3.4 Quest Diagnostics
- 6.3.5 Siemens Healthineers
- 6.3.6 Dragerwerk AG & Co. KGaA
- 6.3.7 Omega Laboratories
- 6.3.8 Premier Biotech Inc.
- 6.3.9 Sciteck Inc.
- 6.3.10 Alfa Scientific Designs
- 6.3.11 QIAGEN N.V.
- 6.3.12 PerkinElmer Inc.
- 6.3.13 OraSure Technologies
- 6.3.14 Neogen Corporation
- 6.3.15 Roche Diagnostics
- 6.3.16 Healgen Scientific LLC
- 6.3.17 Vision Diagnostics Inc.
- 6.3.18 Rochester Regional Health (ACM Global Labs)
- 6.3.19 Nona Scientific Laboratory
- 6.3.20 Applied BioCode Corp.
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-Need Assessment




