PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1906022

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1906022
Pharmaceutical Packaging - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2026 - 2031)
The pharmaceutical packaging market is expected to grow from USD 154.78 billion in 2025 to USD 163.99 billion in 2026 and is forecast to reach USD 219.08 billion by 2031 at 5.95% CAGR over 2026-2031.
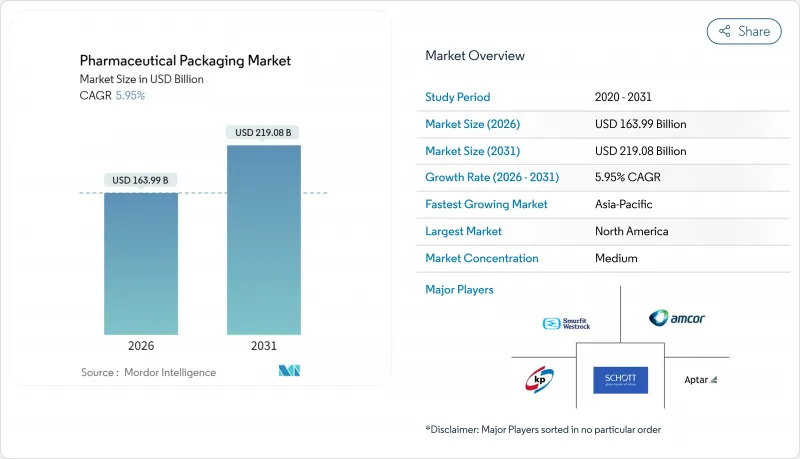
Over the next five years, the increasing demand for biologics, stricter global traceability regulations, and widespread sustainability targets will continue to drive capital investment in new fill-finish lines, high-barrier materials, and circular-ready designs. Demand for flexible pack volumes that match smaller, personalized therapy batches is expected to expand as gene and cell therapies reach commercial scale. North America remains the largest regional contributor, supported by DSCSA-driven serialization, while the Asia-Pacific's sizable 8.96% CAGR reflects rising domestic drug production and broadening health coverage.Material strategies are in flux: plastics still dominate, yet bio-based polymers, aluminum-free blisters, and post-consumer-recycled films are quickly transitioning from pilot to production as EU and US PFAS curbs near enforcement. Meanwhile, price swings in polyethylene, polypropylene, and PET keep margins tight, encouraging longer supplier contracts and vertical integration by larger converters.
Global Pharmaceutical Packaging Market Trends and Insights
Aging population and chronic disease prevalence
Rising median ages drive up long-term therapy volumes, underpinning consistent demand for calendar blisters, large-print labels, and one-hand-open vials that aid adherence among patients with reduced dexterity. Germany's 2024 vaccination shifts, with pneumococcal doses up 23% and meningococcal B up 52%, illustrate broader preventive care uptake among seniors. Packaging suppliers respond with connected packs that log opening events and forward adherence data to care teams. Growth in smart closures and NFC-enabled cartons is expected to intensify as payers link reimbursement to real-world outcomes.
Biologics and injectable pipeline expansion
Prefilled syringes sit at the core of new biologic launches because they simplify self-administration, minimize contamination risks, and reduce waste during the fill-finish process. BD's iDFill syringe embeds RFID for instant verification, while its Neopak XtraFlow design handles viscous formulations that were once vial-only. GMP Annex 1 revisions are accelerating demand for ready-to-use glass tubing and polymer containers that bypass washing and depyrogenation steps, helping CDMOs scale capacity without the need to construct new cleanrooms.
Petro-derivative resin price volatility
Supply disruptions and force majeure events led to a 1.1% increase in PET prices in June 2024, further tightening already narrow converter margins. Pharmaceutical contract material specs restrict rapid grade switches, forcing many converters to absorb cost spikes or renegotiate long contracts. Corrugated shippers also face higher fibre costs, with a USD 70 per-ton increase announced for January 2025.
Other drivers and restraints analyzed in the detailed report include:
- Sustainability-driven material substitution
- Digital traceability mandates (DSCSA, EU-FMD)
- Capital-intensive sterility and validation requirements
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Plastics retained 45.05% of the pharmaceutical packaging market share in 2025, primarily driven by HDPE bottles, PP closures, and PET blisters that strike a balance between cost and barrier needs. Yet the segment's growth moderates as brand owners court circularity objectives. Within the plastics industry, the pharmaceutical packaging market size for PP-based syringes is rising steadily, thanks to break-resistant cyclic olefin options. Glass remains indispensable for light- and moisture-sensitive biologics; Type I borosilicate vials dominate cytotoxic fills, despite their higher weight and shatter risk. Metals play a niche role in aerosol and implantable devices.
Momentum is gathering around bio-attributed resins, recycled PET mid-barrier webs, and paper-based pill bottles, such as Allegheny Health Network's Tully Tube pilot. Developers weigh shelf-life assurance, extractables profiles, and line changeover costs before a wide release, yet early adopters often win procurement tenders from hospitals, incorporating sustainability scoring into vendor audits.
The Pharmaceutical Packaging Market Report is Segmented by Material (Plastics, Glass, Metal, and More), Packaging Level (Primary Packaging, Secondary Packaging, and More), Product Type (Bottles, Prefilled Syringes, Vials and Ampoules, Blister Packs, Caps and Closures, and More), and Geography (North America, South America, Europe, Asia Pacific, and Middle East and Africa). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America accounted for 35.01% of the pharmaceutical packaging market share in 2025, driven by significant investments in biologics and advanced DSCSA serialization mandates. U.S. manufacturers alone are investing USD 160 billion in new fill-finish and API plants through 2025, a trend that drives demand for sterilizable polymers, ready-to-use glass, and high-capacity cold-chain shippers. The region also pilots AI vision systems that scan for sub-100 µm particulates at line speed, lowering recall risk and strengthening brand trust.
Europe balances ambitious sustainability regulations with energy cost pressures. The forthcoming Packaging and Packaging Waste Regulation requires every format to be recyclable by 2030, escalating interest in monomaterial blisters and paper-based pill bottles. Germany's pharmaceutical output slipped 1.5% in 2024, yet its R&D pipelines remain rich in mRNA and gene therapies that require ultra-low-temperature packaging. PFAS restrictions, effective in 2026, force material requalification, offering an early-mover advantage to suppliers with fluorine-free barrier films.
The Asia-Pacific region grows at the fastest rate of 8.73% CAGR through 2031, driven by the expanding CDMO footprints of China and India, as well as the widening of public health coverage. Local regulators align sterility rules with ICH and PIC/S guides, pushing packaging plants to adopt ISO 5 barriers and full-line serialization. Yet the region faces geopolitical headwinds; China's updated Anti-Espionage Law complicates technology transfer for track-and-trace systems, prompting multinationals to diversify sourcing across ASEAN markets. Japanese converters, renowned for their precision molding, secure export orders for COP syringes as global brands hedge against dependence on a single country.
- Amcor plc
- Gerresheimer AG
- Schott AG
- West Pharmaceutical Services Inc.
- AptarGroup Inc.
- Smurfit WestRock
- Becton, Dickinson & Company
- Catalent Inc.
- CCL Industries Inc.
- Klockner Pentaplast Group
- Nipro Corporation
- Vetter Pharma International GmbH
- McKesson Corporation
- FlexiTuff International Ltd.
- W. L. Gore & Associates Inc.
- Stevanato Group
- Corning Incorporated
- Owen-Illinois Inc.
- SGD Pharma
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET LANDSCAPE
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Ageing population and chronic disease prevalence
- 4.2.2 Biologics and injectable pipeline expansion
- 4.2.3 Sustainability-driven material substitution
- 4.2.4 Digital traceability mandates (e.g., DSCSA, EU-FMD)
- 4.2.5 AI-enabled adaptive fill-finish lines
- 4.2.6 Rise of at-home/decentralised trials needing mail-ready packs
- 4.3 Market Restraints
- 4.3.1 Petro-derivative resin price volatility
- 4.3.2 Capital-intensive sterility and validation requirements
- 4.3.3 Looming PFAS/fluoropolymer restrictions in EU and US
- 4.4 Industry Supply Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technological Outlook
- 4.7 Porter's Five Forces Analysis
- 4.7.1 Bargaining Power of Suppliers
- 4.7.2 Bargaining Power of Buyers
- 4.7.3 Threat of New Entrants
- 4.7.4 Threat of Substitutes
- 4.7.5 Degree of Competition
5 MARKET SIZE AND GROWTH FORECASTS (VALUE)
- 5.1 By Material
- 5.1.1 Plastics
- 5.1.1.1 HDPE
- 5.1.1.2 LDPE and LLDPE
- 5.1.1.3 PET
- 5.1.1.4 Other Plastics
- 5.1.2 Glass
- 5.1.2.1 Type I Borosilicate
- 5.1.2.2 Type II Treated Soda-lime
- 5.1.2.3 Type III Soda-lime
- 5.1.3 Metal
- 5.1.4 Paper and Paperboard
- 5.1.5 Biopolymers and Other Materials
- 5.1.1 Plastics
- 5.2 By Packaging Level
- 5.2.1 Primary Packaging
- 5.2.1.1 Bottles
- 5.2.1.2 Prefilled Syringes
- 5.2.1.3 Vials and Ampoules
- 5.2.1.4 Blister Packs
- 5.2.2 Secondary Packaging
- 5.2.2.1 Cartons and Sleeves
- 5.2.2.2 Labels and Inserts
- 5.2.3 Tertiary Packaging
- 5.2.3.1 Corrugated Shippers
- 5.2.3.2 Pallets and Protective Systems
- 5.2.1 Primary Packaging
- 5.3 By Product Type
- 5.3.1 Bottles
- 5.3.2 Prefilled Syringes
- 5.3.3 Vials and Ampoules
- 5.3.4 Blister Packs
- 5.3.5 Caps and Closures
- 5.3.6 Tubes and Pouches
- 5.3.7 Other Product Types
- 5.4 By Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Russia
- 5.4.2.7 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 India
- 5.4.3.3 Japan
- 5.4.3.4 South Korea
- 5.4.3.5 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 Middle East
- 5.4.4.1.1 United Arab Emirates
- 5.4.4.1.2 Saudi Arabia
- 5.4.4.1.3 Turkey
- 5.4.4.1.4 Rest of Middle East
- 5.4.4.2 Africa
- 5.4.4.2.1 South Africa
- 5.4.4.2.2 Nigeria
- 5.4.4.2.3 Egypt
- 5.4.4.2.4 Rest of Africa
- 5.4.4.1 Middle East
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Share Analysis
- 6.4 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products and Services, and Recent Developments)
- 6.4.1 Amcor plc
- 6.4.2 Gerresheimer AG
- 6.4.3 Schott AG
- 6.4.4 West Pharmaceutical Services Inc.
- 6.4.5 AptarGroup Inc.
- 6.4.6 Smurfit WestRock
- 6.4.7 Becton, Dickinson & Company
- 6.4.8 Catalent Inc.
- 6.4.9 CCL Industries Inc.
- 6.4.10 Klockner Pentaplast Group
- 6.4.11 Nipro Corporation
- 6.4.12 Vetter Pharma International GmbH
- 6.4.13 McKesson Corporation
- 6.4.14 FlexiTuff International Ltd.
- 6.4.15 W. L. Gore & Associates Inc.
- 6.4.16 Stevanato Group
- 6.4.17 Corning Incorporated
- 6.4.18 Owen-Illinois Inc.
- 6.4.19 SGD Pharma
7 MARKET OPPORTUNITIES AND FUTURE OUTLOOK
- 7.1 White-space and Unmet-need Assessment




