PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1911268

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1911268
Pharmaceutical Contract Packaging - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2026 - 2031)
pharmaceutical contract packaging market size in 2026 is estimated at USD 20.85 billion, growing from 2025 value of USD 19.16 billion with 2031 projections showing USD 31.86 billion, growing at 8.84% CAGR over 2026-2031.
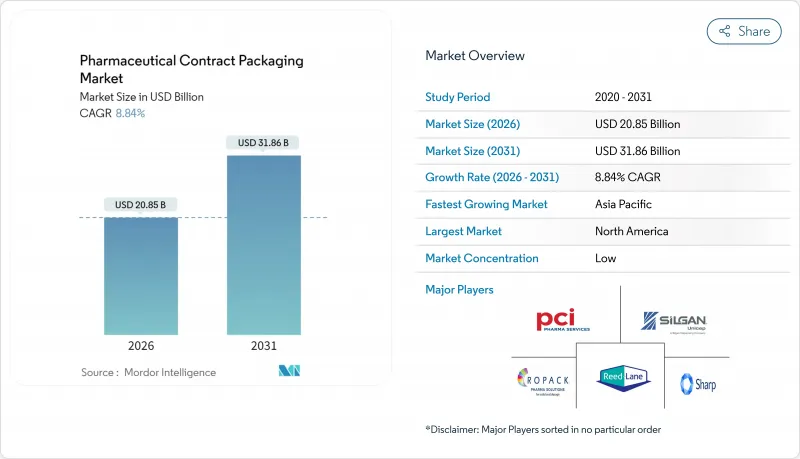
Rising serialization mandates, a booming biologics pipeline and growing preference for integrated CDMO models are steering pharmaceutical companies toward outsourced packaging partners that offer deep technical know-how and capital-intensive capabilities. Demand is particularly strong for sterile primary containers, pre-filled delivery devices and track-and-trace ready secondary packs, while AI-enabled changeover systems are trimming validation cycles and boosting line productivity. Regional near-shoring programs in the United States and the European Union are shifting investment priorities, and Asia-Pacific suppliers are scaling capacity to meet export-oriented generic drug growth.
Global Pharmaceutical Contract Packaging Market Trends and Insights
Serialization Mandates Drive Unprecedented Packaging Transformation
Full DSCSA enforcement in November 2024 forced contract packagers to embed unique serial numbers, barcodes and aggregation data at every packaging level, transforming legacy lines into data-rich operations that manage and reconcile millions of serial numbers daily. Error-rate spikes of 30% in early roll-outs underscored the need for "Serialization 2.0" platforms with open architectures that remove proprietary hardware lock-ins. Investments in edge-to-cloud line controllers accelerated, and vendors now bundle real-time EPCIS data exchange, enabling downstream wholesalers to verify pack authenticity in seconds. As compliance windows tighten in Italy, Canada and Gulf states, global packagers with harmonized systems gain a competitive edge.
Biologics Surge Reshapes Sterile Packaging Infrastructure
World-wide sterile medicinal product output is climbing at 15% CAGR to 2027, and Annex 1 revisions have elevated contamination control to an enterprise-wide priority. Ready-to-use nested vials, ampoules and polymer syringes eliminate glass washing steps, while modern container-closure integrity testing replaces destructive sterility sampling with helium mass-spectrometry and vacuum decay methods. Automated settle-plate changers, such as Syntegon's SPC 1000, cut manual interventions by 80% and drive faster batch-release timelines.
Track-and-Trace Compliance Costs Strain Operational Margins
Divergent serial data formats across markets oblige packagers to operate multi-schema IT stacks, inflating validation and support budgets by up to 20% per multi-market SKU. Italy's February 2025 go-live for EU FMD aggregation illustrates the continuing regulatory drumbeat that forces line retrofits and warehouse upgrades.
Other drivers and restraints analyzed in the detailed report include:
- CDMO Integration Accelerates End-to-End Service Adoption
- Supply-Chain Near-Shoring Transforms Geographic Manufacturing Patterns
- Skilled Labor Shortage Constrains Sterile Manufacturing Expansion
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Primary packaging held a 45.10% share of the pharmaceutical contract packaging market in 2025 and is expanding at 10.05% CAGR as drug-contact materials face tighter leachables and extractables scrutiny.Type I borosilicate glass vials, cyclic olefin polymer cartridges and high-barrier blister films dominate investment plans because they assure chemical compatibility for biologics. Growth momentum also reflects mandatory unit-level serialization, which starts at the primary layer in many jurisdictions.
Sterile container-closure innovation is redefining competitive positioning. Contract packagers now validate package integrity using laser-based headspace analysis and helium mass-spectrometry, reducing destructive testing waste while meeting Annex 1 expectations. Secondary and tertiary services continue to add value through late-stage custom kitting and specialty cold-chain logistics, yet regulatory complexity keeps the revenue center anchored in the primary tier.
Bottles remain the largest format owing to oral solid dosage dominance. However, pre-filled syringes and cartridges show 11.05% CAGR through 2031, making them the fastest-expanding slice of the pharmaceutical contract packaging market. Demand is fuelled by self-injection biologics, pen-injector proliferation and hospital safety mandates that curb needle handling.
Technical advances in cyclic olefin polymer barrels improve drug stability and window clarity, while needle-safety shields and electronic dose counters embed usability features once reserved for device makers. Contract packagers offering integrated plunger placement, nitrogen purging and auto-injector assembly services secure long-term supply agreements with specialty-pharma clients seeking turnkey solutions.
The Pharmaceutical Contract Packaging Market Report is Segmented by Service Type (Primary, Secondary, Tertiary), Packaging Format (Bottles, Vials and Ampoules, and More), Drug Formulation (Solid Dosage, and More), Therapeutic Area (Oncology, Cardiovascular, and More), End-User (Big Pharma, Generics/Biosimilar Companies, and More), and Geography. The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America generated 39.10% of 2025 revenue, supported by FDA serialization mandates, strong biologics pipelines and sizeable near-shoring capital flows. Investments such as Novo Nordisk's USD 4.1 billion Clayton complex embed fill-finish, inspection and final assembly under one roof, shortening supply lines and augmenting domestic resiliency. Canada's sterile-drug corridor in Ontario and Mexico's maquiladora expansions complement US capacity, enabling duty-efficient cross-border flows that satisfy regional content stipulations.
Europe remains an innovation hub, driven by PPWR sustainability rules that accelerate material R&D and by Annex 1 sterile guidelines demanding best-in-class cleanroom performance. Germany's engineering ecosystem anchors high-precision equipment supply, while Italy and France host seasoned fill-finish sites catering to orphan-drug runs. United Kingdom packagers pivot toward advanced therapy applications, leveraging MHRA agility post-Brexit to attract global clinical programs.
Asia-Pacific shows the fastest 10.10% CAGR as Chinese and Indian CDMOs scale capacities that align with generics booms and biosimilar roll-outs. Japan pioneers high-speed robotics in vial filling, South Korea spearheads antibody-drug conjugate projects and Singapore extends tax incentives for cell-therapy facilities. Regional governments nurture GMP convergence under PIC/S, easing multi-country approvals and encouraging cross-border supply networks. Australia and New Zealand contribute niche sterile development services, reinforcing the region's climb up the value chain.
- PCI Pharma Services
- Catalent Inc.
- Sharp Packaging Services
- Almac Group
- Wasdell Group
- Ropack Inc.
- Reed-Lane Inc.
- Jones Healthcare Group
- Recipharm AB
- Tjoapack Netherlands B.V.
- AmeriPac (Veritiv Corp)
- Silgan Unicep
- Nelipak
- Aphena Pharma Solutions
- Central Pharma Contract Packing
- Quantrelle Packaging Solutions
- Variopack GmbH
- Sepha Ltd.
- Assemblies Unlimited
- DaklaPack Group
- Tripak Pharmaceuticals
- MPH Co-Packing
- Southwest Packaging
- MJS Packaging
- Jam Jams Group
- Asiapack (Elanders Group)
- Finishing Services
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET LANDSCAPE
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Serialization mandates and anti-counterfeit regulation surge
- 4.2.2 Biologic and specialty-drug boom amplifying sterile packaging demand
- 4.2.3 CDMO one-stop-shop preference (integrated manufacturing + packaging)
- 4.2.4 Supply-chain near-shoring by Big Pharma in US-EU
- 4.2.5 AI-enabled line-changeover reducing validation time
- 4.3 Market Restraints
- 4.3.1 Evolving global track-and-trace standards raise compliance costs
- 4.3.2 Poly-material sustainability rules squeeze margin on plastics
- 4.3.3 Qualified labor shortage for high-speed sterile filling lines
- 4.4 Supply-Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technological Outlook
- 4.7 Porter's Five Forces Analysis
- 4.7.1 Bargaining Power of Suppliers
- 4.7.2 Bargaining Power of Buyers
- 4.7.3 Threat of New Entrants
- 4.7.4 Threat of Substitutes
- 4.7.5 Intensity of Competitive Rivalry
5 MARKET SIZE AND GROWTH FORECASTS (VALUE)
- 5.1 By Service Type
- 5.1.1 Primary
- 5.1.1.1 Bottles
- 5.1.1.2 Vials and Ampoules
- 5.1.1.3 Blister Packs
- 5.1.2 Secondary
- 5.1.2.1 Cartons
- 5.1.2.2 Labels and Inserts
- 5.1.3 Tertiary
- 5.1.1 Primary
- 5.2 By Packaging Format
- 5.2.1 Bottles
- 5.2.1.1 Plastic Bottles
- 5.2.1.2 Glass Bottles
- 5.2.2 Vials and Ampoules
- 5.2.3 Blister Packs
- 5.2.4 Sachets and Stick Packs
- 5.2.5 Pre-filled Syringes and Cartridges
- 5.2.1 Bottles
- 5.3 By Drug Formulation
- 5.3.1 Solid Dosage
- 5.3.1.1 Tablets
- 5.3.1.2 Capsules
- 5.3.2 Oral Liquids
- 5.3.3 Injectable
- 5.3.3.1 Small-volume Parenterals
- 5.3.3.2 Large-volume Parenterals
- 5.3.1 Solid Dosage
- 5.4 By Therapeutic Area
- 5.4.1 Oncology
- 5.4.2 Cardiovascular
- 5.4.3 CNS
- 5.4.4 Infectious Disease
- 5.4.5 Other Therapeutic Areas
- 5.5 By End-user
- 5.5.1 Big Pharma (>USD 10 bn revenue)
- 5.5.2 Generics/Biosimilar Companies
- 5.5.3 Emerging Biotech and Start-ups
- 5.5.4 CRO/CDMO Partners
- 5.5.5 Others End-user
- 5.6 By Geography
- 5.6.1 North America
- 5.6.1.1 United States
- 5.6.1.2 Canada
- 5.6.1.3 Mexico
- 5.6.2 Europe
- 5.6.2.1 Germany
- 5.6.2.2 United Kingdom
- 5.6.2.3 France
- 5.6.2.4 Italy
- 5.6.2.5 Spain
- 5.6.2.6 Russia
- 5.6.2.7 Rest of Europe
- 5.6.3 Asia-Pacific
- 5.6.3.1 China
- 5.6.3.2 India
- 5.6.3.3 Japan
- 5.6.3.4 South Korea
- 5.6.3.5 Australia and New Zealand
- 5.6.3.6 Rest of Asia-Pacific
- 5.6.4 Middle East and Africa
- 5.6.4.1 Middle East
- 5.6.4.1.1 United Arab Emirates
- 5.6.4.1.2 Saudi Arabia
- 5.6.4.1.3 Turkey
- 5.6.4.1.4 Rest of Middle East
- 5.6.4.2 Africa
- 5.6.4.2.1 South Africa
- 5.6.4.2.2 Nigeria
- 5.6.4.2.3 Egypt
- 5.6.4.2.4 Rest of Africa
- 5.6.4.1 Middle East
- 5.6.5 South America
- 5.6.5.1 Brazil
- 5.6.5.2 Argentina
- 5.6.5.3 Rest of South America
- 5.6.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Share Analysis
- 6.4 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products and Services, and Recent Developments)
- 6.4.1 PCI Pharma Services
- 6.4.2 Catalent Inc.
- 6.4.3 Sharp Packaging Services
- 6.4.4 Almac Group
- 6.4.5 Wasdell Group
- 6.4.6 Ropack Inc.
- 6.4.7 Reed-Lane Inc.
- 6.4.8 Jones Healthcare Group
- 6.4.9 Recipharm AB
- 6.4.10 Tjoapack Netherlands B.V.
- 6.4.11 AmeriPac (Veritiv Corp)
- 6.4.12 Silgan Unicep
- 6.4.13 Nelipak
- 6.4.14 Aphena Pharma Solutions
- 6.4.15 Central Pharma Contract Packing
- 6.4.16 Quantrelle Packaging Solutions
- 6.4.17 Variopack GmbH
- 6.4.18 Sepha Ltd.
- 6.4.19 Assemblies Unlimited
- 6.4.20 DaklaPack Group
- 6.4.21 Tripak Pharmaceuticals
- 6.4.22 MPH Co-Packing
- 6.4.23 Southwest Packaging
- 6.4.24 MJS Packaging
- 6.4.25 Jam Jams Group
- 6.4.26 Asiapack (Elanders Group)
- 6.4.27 Finishing Services
7 MARKET OPPORTUNITIES AND FUTURE OUTLOOK
- 7.1 White-space and Unmet-Need Assessment




