PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1850110

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1850110
Brain Monitoring - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The brain monitoring market size stands at USD 7.23 billion in 2025 and is on track to reach USD 9.84 billion by 2030, reflecting a 6.36% CAGR.
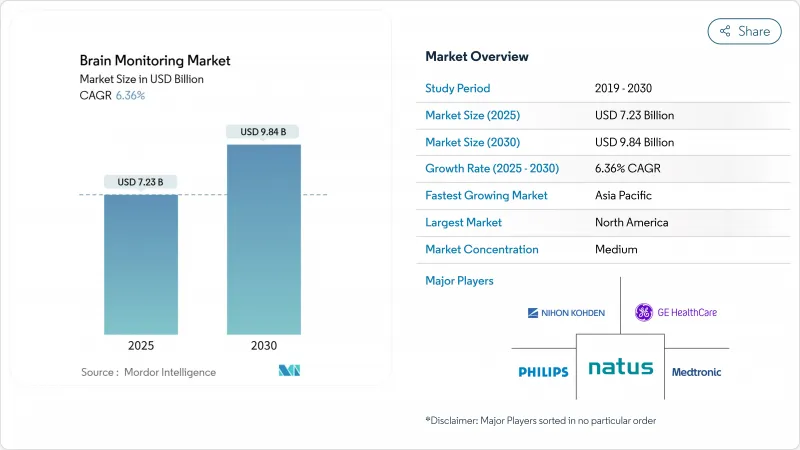
Demand is expanding as neurological disorders climb to the top of the global disease burden, so providers are investing in faster, more portable diagnostic tools. Advancements in artificial intelligence (AI) are improving multimodal data interpretation, while minimally invasive and wearable sensors are moving routine monitoring into emergency rooms, rehabilitation centers, and homes. Fixed systems still dominate hospital spending, yet growth is strongest in compact and cloud-connected devices that support tele-neurology and decentralized trials. Vendors are refocusing from standalone hardware toward integrated software-as-a-medical-device (SaMD) platforms that automate data analysis, ease staffing gaps, and create recurring revenue from analytics subscriptions.
Global Brain Monitoring Market Trends and Insights
Escalating Prevalence of Neurological Disorders
Cases of stroke, dementia, epilepsy, and neuropathies now affect more than 3 billion individuals-43% of the global population-over 18% jump since 1990. These conditions generate 443 million years of healthy life lost each year, intensifying pressure on health systems. Growing longevity worsens exposure to age-linked illnesses such as Alzheimer's, while metabolic diseases like diabetes triple the incidence of neuropathy. The widening gap between patient demand and neurologist supply pushes hospitals to adopt automated monitoring that triages high-risk cases and feeds data into centralized interpretation hubs. Over the next decade, universal screening programs and community clinics are expected to rely on lower-cost neural sensors to catch early deterioration, sustaining long-run expansion of the brain monitoring market.
AI-Enabled Multimodal Analytics for Enhanced Diagnostic Accuracy
Machine-learning algorithms now integrate EEG, imaging, hemodynamics, and clinical scores to predict mortality or poor outcomes in traumatic brain injury with up to 95.6% accuracy. FDA-cleared platforms such as Ceribell Clarity deliver bedside electrographic status-epilepticus detection within minutes, freeing critical-care teams from manual review. Hospitals in North America and Europe are embedding these SaMD modules into existing monitors, creating service contracts that bundle cloud analytics with consumables. As reimbursement codes recognize algorithm-assisted interpretation, adoption in urban Asia-Pacific centers is accelerating. Over the medium term, richer data pipelines will shorten decision times in stroke and intensive-care units, lifting utilization across the brain monitoring market.
High Purchase and Upkeep Costs for Advanced Modalities
Magnetoencephalography and fMRI systems can exceed USD 3 million, with annual service contracts adding 10-15% of list price. Even mainstream EEG rigs require consumables and calibration that strain hospital operating budgets. Replacement cycles accelerate as vendors launch AI-ready models, prompting finance committees to defer purchases in price-sensitive markets. Open-source wearable fNIRS prototypes from academic labs promise relief, yet certification remains years away, so cost remains a mid-term drag on the brain monitoring market.
Other drivers and restraints analyzed in the detailed report include:
- Proliferation of Wearable and Minimally Invasive Brain Sensors
- Investment Surge in Neuro-Critical-Care Infrastructure
- Global Shortage of Trained Neuro-Technologists
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
EEG systems generated the most significant slice of the brain monitoring market in 2024 by capturing 30.44% revenue. Hospitals rely on them for seizure diagnostics, sedation depth checks, and cerebral ischemia surveillance. Portable rapid-EEG carts now transform emergency workflows by delivering seizure detection within 10 minutes at the bedside, boosting device turnover. Accessories-caps, dry electrodes, cables-grow fastest at an 8.11% CAGR because every new unit drives recurring consumable demand. Flexible polymer electrodes improve comfort and cut setup time, widening EEG use in pediatrics and tele-monitoring. As more hospitals standardize on disposable caps to limit infection risk, the accessories sub-segment will outpace the overall brain monitoring market.
Non-invasive modalities such as scalp EEG, fNIRS, and transcranial Doppler controlled 73.78% of 2024 revenue thanks to lower risk and simpler staffing. Their appeal broadens as algorithm-enhanced systems approximate invasive accuracy. Yet critical-care units still depend on intracranial pressure (ICP) probes and depth electrodes for refractory cases. These invasive tools expand at a 6.95% CAGR as new nanomembrane sensors enter trials, delivered through blood vessels and eliminating skull penetration. Over time, hybrid solutions may blur categories further, sustaining growth in both cohorts of the brain monitoring market.
The Brain Monitoring Market Report is Segmented by Product Type (Magnetoencephalograph (MEG) Systems, Accessories [Electrodes and More], and More), Procedure (Invasive Monitoring and More), Modality (Fixed/Standalone Systems and More), Application (Traumatic Brain Injury and More), End-User (Hospitals and More), and Geography (North America, Europe, Asia-Pacific, and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America held 37.75% of global sales in 2024, supported by robust insurance coverage, NIH grants, and a dense network of level-one trauma centers. The brain monitoring market size in the region is expanding at a steady 6.12% CAGR as hospitals refresh fleets to AI-enabled models and outpatient providers embrace remote EEG kits. Europe follows with mature yet methodical procurement; growth runs at 5.87% as budget oversight slows but does not halt adoption. Noteworthy is a handheld ocular laser that detects TBI biomarkers during the golden hour after injury, a potential standard of care in ambulances.
Asia-Pacific posts the quickest gains at an 8.59% CAGR. China funds stroke centers in tier-2 cities; Japan pilots insurance codes for home EEG; India's private hospitals import rapid-EEG carts for emergency departments. Given that 80% of neurological deaths occur in low- and middle-income countries, regional demand for affordable, low-maintenance devices remains high. Local manufacturers have begun supplying cost-effective alternatives, reinforcing supply-chain resilience in the brain monitoring market.
The Middle East & Africa and South America expand from smaller bases as Gulf states build neuro-critical centers and Brazilian hospitals join telestroke networks. However, currency volatility and procurement hurdles temper acceleration. Multilateral lenders and public-private partnerships are expected to underwrite pilot programs that demonstrate clinical and economic value, creating footholds for future market growth.
- Abbott Laboratories
- Advanced Brain Monitoring
- Boston Scientific
- BrainScope Company Inc.
- Cadwell
- Cerenion
- Compumedics
- Elekta
- EMOTIV Inc.
- GE Healthcare
- Koninklijke Philips
- Masimo
- Medtronic
- Mespere LifeSciences
- Natus Medical
- Neurable Inc.
- NeuraSignal, Inc.
- NeuroSky Inc.
- Nihon Kohden
- Siemens Healthineers
- Zeto, Inc.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Escalating prevalence of neurological disorders
- 4.2.2 AI-enabled multimodal analytics that enhance diagnostic accuracy
- 4.2.3 Proliferation of wearable and minimally invasive brain sensors
- 4.2.4 Investment surge in neuro-critical-care infrastructure
- 4.2.5 Brain-focused drug trials requiring objective monitoring biomarkers
- 4.2.6 Accelerated approvals for software-as-a-medical-device modules
- 4.3 Market Restraints
- 4.3.1 High purchase and upkeep costs for advanced modalities
- 4.3.2 Global shortage of trained neuro-technologists
- 4.3.3 Interoperability and cybersecurity risks in connected devices
- 4.3.4 Uncertain reimbursement for ambulatory monitoring solutions
- 4.4 Supply-Chain Analysis
- 4.5 Technological Outlook
- 4.6 Porter's Five Forces Analysis
- 4.6.1 Threat of New Entrants
- 4.6.2 Bargaining Power of Buyers / Consumers
- 4.6.3 Bargaining Power of Suppliers
- 4.6.4 Threat of Substitute Products & Services
- 4.6.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value)
- 5.1 By Product Type
- 5.1.1 Magnetoencephalograph (MEG) Systems
- 5.1.2 Electroencephalograph (EEG) Systems
- 5.1.3 Transcranial Doppler (TCD) Ultrasound
- 5.1.4 Cerebral Oximeters
- 5.1.5 Magnetic Resonance Imaging (MRI) Devices
- 5.1.6 Intracranial Pressure (ICP) Monitors
- 5.1.7 Computerized Tomography (CT) Devices
- 5.1.8 Positron Emission Tomography (PET) Devices
- 5.1.9 Accessories
- 5.1.9.1 Electrodes
- 5.1.9.2 Sensors
- 5.1.9.3 Cables
- 5.1.9.4 Gels & Pastes
- 5.1.9.5 Other Accessories
- 5.1.10 Other Brain Monitoring Devices
- 5.2 By Procedure
- 5.2.1 Invasive Monitoring
- 5.2.2 Non-invasive Monitoring
- 5.3 By Modality
- 5.3.1 Fixed / Standalone Systems
- 5.3.2 Portable & Wearable Systems
- 5.4 By Application
- 5.4.1 Traumatic Brain Injury
- 5.4.2 Stroke
- 5.4.3 Epilepsy
- 5.4.4 Parkinson's Disease
- 5.4.5 Alzheimer's & Other Dementias
- 5.4.6 Sleep Disorders
- 5.4.7 Other Neurological Conditions
- 5.5 By End-User
- 5.5.1 Hospitals
- 5.5.2 Diagnostic & Imaging Centers
- 5.5.3 Ambulatory Surgical & Specialty Clinics
- 5.5.4 Home-care Settings & Tele-neurology Platforms
- 5.5.5 Academic & Research Institutes
- 5.6 By Geography
- 5.6.1 North America
- 5.6.1.1 United States
- 5.6.1.2 Canada
- 5.6.1.3 Mexico
- 5.6.2 Europe
- 5.6.2.1 Germany
- 5.6.2.2 United Kingdom
- 5.6.2.3 France
- 5.6.2.4 Italy
- 5.6.2.5 Spain
- 5.6.2.6 Rest of Europe
- 5.6.3 Asia-Pacific
- 5.6.3.1 China
- 5.6.3.2 India
- 5.6.3.3 Japan
- 5.6.3.4 Australia
- 5.6.3.5 South Korea
- 5.6.3.6 Rest of Asia-Pacific
- 5.6.4 Middle East & Africa
- 5.6.4.1 GCC
- 5.6.4.2 South Africa
- 5.6.4.3 Rest of Middle East & Africa
- 5.6.5 South America
- 5.6.5.1 Brazil
- 5.6.5.2 Argentina
- 5.6.5.3 Rest of South America
- 5.6.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Product Portfolio Analysis
- 6.3 Market Share Analysis
- 6.4 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.4.1 Abbott Laboratories
- 6.4.2 Advanced Brain Monitoring Inc.
- 6.4.3 Boston Scientific Corporation
- 6.4.4 BrainScope Company Inc.
- 6.4.5 Cadwell Industries Inc.
- 6.4.6 Cerenion Oy
- 6.4.7 Compumedics Limited
- 6.4.8 Elekta AB
- 6.4.9 EMOTIV Inc.
- 6.4.10 GE Healthcare
- 6.4.11 Koninklijke Philips N.V.
- 6.4.12 Masimo Corporation
- 6.4.13 Medtronic PLC
- 6.4.14 Mespere LifeSciences
- 6.4.15 Natus Medical Incorporated
- 6.4.16 Neurable Inc.
- 6.4.17 NeuraSignal, Inc.
- 6.4.18 NeuroSky Inc.
- 6.4.19 Nihon Kohden Corporation
- 6.4.20 Siemens Healthineers AG
- 6.4.21 Zeto, Inc.
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-need Assessment




