PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1685691

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1685691
Urinary Tract Infection Therapeutics - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
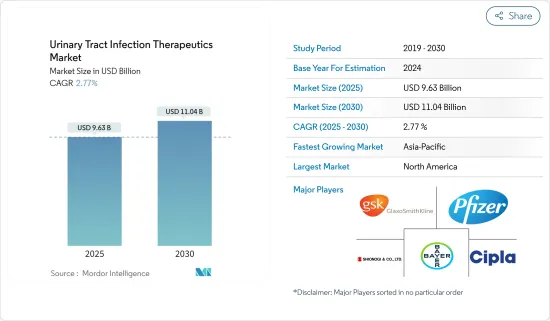
The Urinary Tract Infection Therapeutics Market size is estimated at USD 9.63 billion in 2025, and is expected to reach USD 11.04 billion by 2030, at a CAGR of 2.77% during the forecast period (2025-2030).
The unprecedented increase in coronavirus-infected patients had reduced access to other primary care services and resulted in a significant drop in non-COVID-19-related diagnoses, such as urinary tract infection. The weekly rate of UTI diagnosis fell slightly in 2020, which may have slowed the industry's growth in recent months. According to a study published in the ADIAN Journal in December 2020, a significant decrease in urinary tract infection (UTI) diagnoses due to the COVID-19 pandemic raises concern. When people with COVID-19 were infected with SARS-CoV-2, the pandemic changed primary care dramatically. This resulted in fewer patients seeking primary care services and fewer diagnoses, including UTIs. Between March 30 and April 24, 2020, the weekly rate of UTI diagnosis per 100,000 population fell from an average of 30-35 to less than 10 in England. Since April, the rate has risen by 50% of the usual rate. The increase in the rate of UTI diagnosis has positively impacted market growth. Certain factors driving the market growth include the increasing prevalence of diabetes and kidney stones and the launch of combination drugs.
According to the American Academy of Family Physicians (AAFP), in March 2020, by Leonardo Ferreira, kidney stones were a common ailment, with an annual incidence of eight instances per 1,000 persons. As per the same source, around 13% of men and 7% of women may develop a kidney stone during their lifetime, and the total incidence of urinary retention in the US is 4.5 to 6.8 per 1,000 men per year. Moreover, according to the International Federation of Diabetics report in 2021, diabetes affected approximately 463 million persons aged 20 to 79 years worldwide in 2020. This number is anticipated to climb to 643 million by 2030 and 700 million by 2045. In most countries, the proportion of people with Type 2 diabetes is increasing, and diabetes affects 79% of adults in low- and middle-income countries. Urinary tract infections can be especially troublesome for people with diabetes because sugar in the urine serves as a breeding ground for bacteria. Therefore, with the increasing prevalence of diabetes and kidney stones, the number of cases of urinary tract infection (UTI) increases, increasing the demand for drugs, thus, driving the global urinary tract infection therapeutics market.
Additionally, the launch of more efficient combination drugs and the increasing geriatric population is expected to boost the growth of the urinary tract infection therapeutics market. For instance, in February 2020, Allecra, a French pharmaceutical company, announced Exblifep, a combination of enmetazobactam, a novel extended-spectrum beta-lactamase inhibitor, and cefepime, a fourth-generation cephalosporin that met primary endpoints in a clinical trial for complicated UTIs.
However, adverse events associated with the use of medication and the lack of awareness about the prevalence of UTIs in developing and underdeveloped countries are restraining the market's growth.
Urinary Tract Infection (UTI) Therapeutics Market Trends
Complicated UTIs Segment Expected to Hold a Major Share in the Urinary Tract Infection Therapeutics Market
The prevalence of complicated UTIs is expected to increase in the future, owing to the rise in drug-resistant bacteria and excessive use of antibiotics. A vast majority of physicians prescribe quinolones to treat complicated UTI cases. Cephalosporin is the second-most common drug prescribed for complicated UTI cases.
According to an article published in the International Journal of Molecular medicine in 2020, kidney stone disease, also known as nephrolithiasis or urolithiasis, is one of medicine's oldest diseases. It is estimated that 11% of people will develop kidney stones at some point in their lives, and the prevalence and incidence of kidney stones are increasing worldwide. However, the overall prevalence of urolithiasis was 11.2%, with 48.8% of those surveyed having a first-degree relative with the disease. Males were 1.8 times more likely than females to have urolithiasis. Overall, the prevalence of complicated UTIs is set to increase during the forecast period, mainly owing to the increasing bacterial resistance in UTI cases and the rise in recurrence rate for UTIs.
However, increasing approvals from the US Food and Drug Administration and product launches by key players are expected to boost the market. For instance, in July 2019, the US Food and Drug Administration approved Merck and Company's Recarbrio (imipenem, cilastatin, and relebactam), an antibacterial drug product to treat adults with complicated urinary tract infections (cUTI) and complicated intra-abdominal infections (cIAI).
Thus, all aforementioned factors such as rising prevalence of cUTIs and increasing product launches are expected to boost the segment over the forecast period.
North America Expected to Hold Significant Market Share in the Forecast Period
The region is experiencing a drastic increase in innovations related to diagnostic methodologies used for UTIs. According to the article published in Infectious Diseases Society of America in November 2020, by Pranita D. Tamma, during a routine checkup for a UTI, a woman was observed to be infected by E. coli that showed resistance to the last-resort antibiotic, Colistin. The discovery of Colistin-resistant bacteria was considered a major issue. In addition to that, the CDC, in collaboration with other organizations, developed guidelines for preventing catheter-associated UTIs and other healthcare-associated infections in the US. Furthermore, the Queensland Pediatric Factsheet 2019 estimated that approximately 1 in 10 girls and 1 in 50 boys would suffer from a urinary tract infection by seven years of age. Infections in children under one year are more common in boys, but in older children, infections are more common in girls.
Additionally, according to a study published in Therapeutic Advances in Urology in May 2019 titled, "An Introduction to the Epidemiology and Burden of Urinary Tract Infections," urinary tract infections (UTIs) are the most common outpatient infections in adult women, with a lifetime prevalence of 50-60%. UTIs are a substantial societal and personal burden, with UTIs accounting for a significant number of medical visits in the US each year. This condition is expected to drive the market by increasing the sales of urinary tract infection medicines.
Furthermore, research and development of novel classes of antibiotics for urinary tract infections are expected to aid in market growth. In August 2020, for example, researchers at California Polytechnic State University announced the development of a new class of antibiotics for urinary tract infections that target bacterial iron acquisition.
Thus, all aforementioned factors are expected to boost the market in the region over the forecast period.
Urinary Tract Infection (UTI) Therapeutics Industry Overview
The urinary tract infection therapeutics market is fragmented and competitive and consists of a number of major players. Some of the companies currently dominating the market are AstraZeneca, Bayer AG, Cipla Inc., GlaxoSmithKline PLC, Shionogi & Co. Ltd, Novartis AG, and Pfizer, among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Prevalence of Diabetes and Kidney Stones
- 4.2.2 Launch of Combination Drugs
- 4.3 Market Restraints
- 4.3.1 Adverse Effects Associated with the Use of Medication
- 4.3.2 Lack of Awareness in Developing and Underdeveloped Countries
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 Drug
- 5.1.1 Penicillin and Combinations
- 5.1.2 Quinolones
- 5.1.3 Cephalosporin
- 5.1.4 Azoles and Amphotericin B
- 5.1.5 Nitrofurans
- 5.1.6 Other Drugs (Aminoglycoside Antibodies, Sulphonamides, Tetracycline, etc.)
- 5.2 Indication
- 5.2.1 Complicated UTI
- 5.2.2 Uncomplicated UTI
- 5.2.3 Other Indications (Recurring Complicated UTI, Neurogenic Bladder Infection, etc.)
- 5.3 Geography
- 5.3.1 North America
- 5.3.1.1 US
- 5.3.1.2 Canada
- 5.3.1.3 Mexico
- 5.3.2 Europe
- 5.3.2.1 Germany
- 5.3.2.2 UK
- 5.3.2.3 France
- 5.3.2.4 Italy
- 5.3.2.5 Spain
- 5.3.2.6 Rest of Europe
- 5.3.3 Asia-Pacific
- 5.3.3.1 China
- 5.3.3.2 Japan
- 5.3.3.3 India
- 5.3.3.4 Australia
- 5.3.3.5 South Korea
- 5.3.3.6 Rest of Asia-Pacific
- 5.3.4 Middle-East and Africa
- 5.3.4.1 GCC
- 5.3.4.2 South Africa
- 5.3.4.3 Rest of Middle-East and Africa
- 5.3.5 South America
- 5.3.5.1 Brazil
- 5.3.5.2 Argentina
- 5.3.5.3 Rest of South America
- 5.3.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 AstraZeneca
- 6.1.2 Bayer AG
- 6.1.3 Cipla Inc.
- 6.1.4 GlaxoSmithKline PLC
- 6.1.5 Shionogi & Co. Ltd
- 6.1.6 Novartis AG
- 6.1.7 Pfizer
- 6.1.8 Merck & Co. Inc
- 6.1.9 Bristol-Myers Squibb Company
- 6.1.10 Almirall SA
- 6.1.11 Dr. Reddys Laboratories Ltd
- 6.1.12 Allergan
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




