PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1433473

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1433473
Global Tuberculosis Diagnostics - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029)
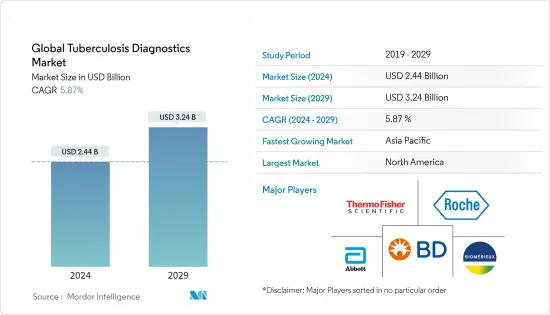
The Global Tuberculosis Diagnostics Market size is estimated at USD 2.44 billion in 2024, and is expected to reach USD 3.24 billion by 2029, growing at a CAGR of 5.87% during the forecast period (2024-2029).
The COVID-19 pandemic had a significant impact on the healthcare systems, with significant consequences not only for COVID-19-infected patients but also for others, resulting in the cancellation of diagnosis and treatment services to manage and reserve resources, and many hospitals are experiencing a shortage of professionals to assist with these diagnostic procedures. The COVID-19 restrictions imposed by the government have impacted the market growth. For instance, according to the data published by the World Health Organization, in March 2021, titled 'Impact of the COVID-19 pandemic on TB detection and mortality in 2020', it has been observed that an estimated 1.4 million fewer persons underwent TB treatment in 2020 than in 2019, a 21% decrease from 2019. Additionally, as per an article published by the International Journal of Tuberculosis and Lung Disease, in June 2021, titled 'The impact of COVID-19 on TB: a review of the data', it has been observed that the provision of tuberculosis services has been hampered with high TB burden due to inadequate capacity and equipment, restrictions on migration (affecting health care staff, supplies, and stock), and resource reallocation. Also, tuberculosis patients have struggled to get TB services, either due to fear of SARS-CoV-2 infection, fear of stigma, mobility restrictions, shortened health facility operating hours, or reduced financial resources to pay for care or transportation. As a result, TB diagnosis, treatment, and prevention have been impacted globally. Thus, COVID-19 has negatively impacted the market growth during the pandemic.
Certain factors that are propelling the market growth are the increasing burden of tuberculosis and multidrug-resistant tuberculosis (MDR-TB), increasing research and development investments and intensive product pipelines and increasing government initiatives, and rising awareness in the emerging markets.
The rising incidences of tuberculosis and multi-drug resistant tuberculosis (MDR-TB) is the key factor driving the market growth. For instance, according to the Global Tuberculosis Report 2021 published by the World Health Organization, in October 2021, most of the tuberculosis cases were found in the World Health Organization (WHO) regions of South-East Asia (43%), Africa (25%), and the Western Pacific (18%), and lesser numbers of tuberculosis cases were found in Eastern Mediterranean (8.3%), and Europe (2.3%). Additionally, according to the data published by the World Health Organization (WHO), in October 2021, about 1.5 million people worldwide died because of tuberculosis (TB), in 2020, and approximately 10 million people fell ill with tuberculosis globally. Thus, the rising burden of tuberculosis among the population increases the need for better diagnostic tests and treatment, which in turn is anticipated to propel the market growth over the forecast period.
Additionally, according to an article published by the Journal of Family Medicine and Primary Care, in January 2022, titled 'Status of drug-resistant tuberculosis among patients attending a tuberculosis unit of West Bengal', it has been observed that the prevalence of tuberculosis and multidrug-resistant tuberculosis (MDR-TB) is highest in India and ranks first in the global ranking of detecting new cases each year. In addition, as per the same source, an estimated overall incidence of tuberculosis in India was 193 per lakh people, in 2020. Similarly, as per the latest report of the Government of India published in March 2021, there were an estimated 1,24,000 instances of MDR/RR-TB in India (9.1 lakh people), in 2021. Thus, the rising burden of MDR-TB cases among the population is expected to increase the demand for early diagnosis of the disease for further treatment, which in turn is anticipated to augment the market growth over the forecast period.
Furthermore, the growing company's focus on research and development activities and intensive product pipelines are also contributing to the growth of the market. For instance, in October 2021, Qiagen launched the QIAreach QuantiFERON-TB test, built on the proven QuantiFERON-TB Gold Plus technology for tuberculosis (TB) infection. Also, in July 2020, The Foundation for Innovative New Diagnostics (FIND) and Cepheid, Inc, launched a new drug-resistant tuberculosis profiling test, Xpert MTB/XDR, that can be used to empower clinicians to quickly prescribe treatment regimens for extensively drug-resistant TB (XDR-TB). The test can give a result in 90 minutes.
However, the high costs associated with tuberculosis diagnostics and low coverage or absence of insurance in the emerging markets are some of the factors that are hindering the market growth over the forecast period.
Tuberculosis Diagnostics Market Trends
Nucleic Acid Testing Segment Expects to Register a High CAGR During the Forecast Period
The Nucleic Acid Testing segment is expected to witness significant growth in the market owing to the factors such as the rising adoption of nucleic acid testing and advantages offered by nucleic acid testing such as better accuracy than other microscopy tests and providing faster results, over other tests.
The rising adoption of nucleic acid testing for detecting tuberculosis is the key factor driving the market growth. For instance, according to an article published by the Journal of Clinical Microbiology, in September 2020, titled 'Advances in Molecular Diagnosis of Tuberculosis', the World Health Organization (WHO) has recommended the use of molecular nucleic acid amplification tests (NAATs) tests for tuberculosis, as these are more accurate at detecting the disease, particularly in patients with the paucibacillary disease and HIV-positive individuals. This is expected to increase the segment growth over the forecast period.
In addition, the data published by the World Health Organization, in February 2021, stated that the World Health Organization has updated the policies on molecular assays used for the diagnosis of TB and drug resistance, in the December 2020 meeting. The meeting covered the high diagnostic accuracy of 3 new classes of technologies such as moderate complexity automated Nucleic Acid Amplification Tests (NAATs), for detection of TB and resistance to rifampicin and isoniazid; low complexity automated NAATs for the detection of resistance to isoniazid and second-line anti-TB agents and high complexity hybridization-based NAATs for the detection of resistance to pyrazinamide.
Furthermore, the advantages offered by nucleic acid testing over other tests for detecting tuberculosis are expected to propel market growth over the forecast period. For instance, according to the 2022 data published by the State of Rhode Island Department of Health, it has been observed that Rhode Island State Health Laboratory (RISHL) utilizes the Cepheid the Xpert MTB/RIF assayfor Nucleic Acid Amplification Tests (NAATs), an automated molecular diagnostic test that is used to detect the presence of Mycobacterium tuberculosis complex (MTB-complex) DNA in respiratory specimens from both adults and children. In addition, as per the same source, the NAAT can be performed directly on sputum and other respiratory specimens and results can be delivered to the physician 24 to 48 hours after specimen receipt.
Therefore, the due to aforementioned factors the studied market is expected to increase over the forecast period.
North America Dominates the Market and Expects to do Same Over the Forecast Period
North America is expected to dominate the tuberculosis diagnostic market owing to the factors such as the rising number of tuberculosis cases in the region, the presence of well-established healthcare infrastructure, and high healthcare spending.
The rising incidence of tuberculosis in the region is the key factor driving the market growth. For instance, according to the Centers for Disease Control and Prevention's National Tuberculosis Surveillance System (NTSS), a total of 7,860 tuberculosis cases were reported in the 50 United States and the District of Columbia (DC), in 2021. The national incidence of reported tuberculosis cases (cases per 100,000 people) increased by 9.4% in 2021 as compared to 2020. In addition, as per the same source, the increase in tuberculosis cases among the population in 2021 was due to the delayed diagnosis of cases in persons with symptom onset during 2020. Also, in 2021, some of the countries with the highest number of tuberculosis cases were California (1,750), Texas (991), Florida (499), Pennsylvania (166), Ohio (149), and Arizona (129). Thus, the growing burden of tuberculosis among the population is expected to raise the demand for better diagnostic tests which is expected to increase the market growth over the forecast period.
In addition, the rising healthcare spending in the region is expected to increase the company's activities for developing advanced treatments, thereby propelling the market growth. For instance, according to the Organization for Economic Co-operation and Development (OECD), in June 2022, United States healthcare spending, in 2021 was 17.8% of the total GDP of the country. Additionally, as per the data published by the Centers for Medicare & Medicaid Services, in March 2022, titled 'CMS Office of the Actuary Releases 2021-2030 Projections of National Health Expenditures', it has been observed that the annual growth in national health spending is expected to average 5.1% over 2021-2030. In addition, the National Health Spending in 2020 was USD 4.1 trillion and it is projected to reach USD 6.8 trillion by 2030.
Therefore, the due to aforementioned factors the studied market is expected to increase over the forecast period.
Tuberculosis Diagnostics Industry Overview
The tuberculosis market is fairly competitive with the presence of a large number of players in the market. The new product launches, collaborations, mergers and acquisitions, and regional expansions are the key strategic initiatives undertaken by the industry players. Some of the players in the market are Abbott, Becton, Dickinson and Company, BioMerieux SA, Cepheid, Qiagen, and F. Hoffmann-La Roche AG, among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Burden of Tuberculosis and Multidrug-resistant Tuberculosis (MDR-TB)
- 4.2.2 Increasing R&D Investments and Intensive Product Pipelines
- 4.2.3 Increasing Government Initiatives and Rising Awareness in the Emerging Markets
- 4.3 Market Restraints
- 4.3.1 High Costs Associated with Tuberculosis Diagnostics
- 4.3.2 Low Coverage or Absence of Insurance in the Emerging Markets
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value in USD million)
- 5.1 By Diagnostic Test Type
- 5.1.1 Radiographic Test
- 5.1.2 Laboratory Test
- 5.1.3 Nucleic Acid Testing
- 5.1.4 Cytokine Detection Test
- 5.1.5 Drug Resistance Test
- 5.1.6 Other Diagnostic Test Types
- 5.2 By End User
- 5.2.1 Hospital/Clinics
- 5.2.2 Diagnostics/Research Laboratory
- 5.2.3 Other End Users
- 5.3 Geography
- 5.3.1 North America
- 5.3.1.1 United States
- 5.3.1.2 Canada
- 5.3.1.3 Mexico
- 5.3.2 Europe
- 5.3.2.1 Germany
- 5.3.2.2 United Kingdom
- 5.3.2.3 France
- 5.3.2.4 Italy
- 5.3.2.5 Spain
- 5.3.2.6 Rest of Europe
- 5.3.3 Asia-Pacific
- 5.3.3.1 China
- 5.3.3.2 Japan
- 5.3.3.3 India
- 5.3.3.4 Australia
- 5.3.3.5 South Korea
- 5.3.3.6 Rest of Asia-Pacific
- 5.3.4 Middle East & Africa
- 5.3.4.1 GCC
- 5.3.4.2 South Africa
- 5.3.4.3 Rest of Middle East and Africa
- 5.3.5 South America
- 5.3.5.1 Brazil
- 5.3.5.2 Argentina
- 5.3.5.3 Rest of South America
- 5.3.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Abbott Laboratories
- 6.1.2 Becton, Dickinson and Company
- 6.1.3 BioMerieux SA
- 6.1.4 Cepheid
- 6.1.5 F. Hoffmann-La Roche AG
- 6.1.6 Hain Lifescience GmbH
- 6.1.7 Hologic Corporation
- 6.1.8 Qiagen
- 6.1.9 AdvaCare Pharma
- 6.1.10 Thermo Fisher Scientific Inc.
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




