PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1444441

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1444441
Global Migraine Therapeutics - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029)
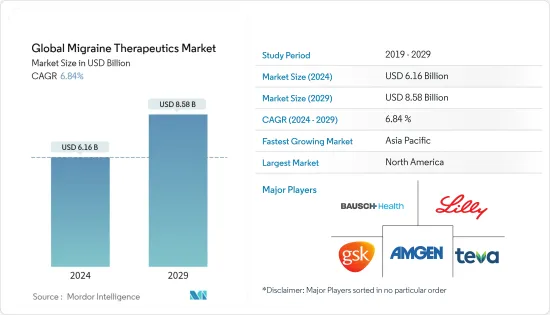
The Global Migraine Therapeutics Market size is estimated at USD 6.16 billion in 2024, and is expected to reach USD 8.58 billion by 2029, growing at a CAGR of 6.84% during the forecast period (2024-2029).
COVID-19 was a global public health crisis, and it impacted almost every industry, and its long-term effects were expected to affect industry growth over the forecast period. According to the study titled "Impact of COVID-19 pandemic on migraine management in the United States: insights from migraine tracking app users" published in BMC neurology in September 2021, although the number of migraine attacks decreased, the proportion of stress-related migraine attacks peaked at 53% during COVID-19. Also, the study titled "COVID-19 and Headache Medicine: A Narrative Review of NonSteroidal AntiInflammatory Drug (NSAID) and Corticosteroid Use" published in July 2021, reported that there may be an increase in the number of patients with headaches as their primary complaint during the COVID19 pandemic. Hence, COVID-19 had a significant impact on the migraine therapeutics market.
Growing awareness about migraine and treatment options, rising R&D spending leading to massive pipeline products, and an increase in the prevalence of migraines with high unmet needs are all contributing to the global growth of the migraine therapeutics market.
The various strategic activities by the key market players, such as product launches and product approvals, as well as mergers and acquisitions, boost the market over the forecast period. For instance, in March 2022, AbbVie reported a positive phase 3 progress trial evaluating atogepant (QULIPTA in the United States) for the preventive treatment of chronic migraine in adults. It is an oral calcitonin gene-related peptide (CGRP) receptor antagonist (gepant). In the trial, the drug met its primary endpoint of a statistically significant reduction from baseline in mean monthly migraine days compared to placebo, for both the 60 mg once daily (QD) and 30 mg twice daily (BID) doses, across the 12-week treatment period.
Additionally, in September 2021, the United States Food and Drug Administration (FDA) approved QULIPTA (atogepant) to AbbVie for the prevention of episodic migraine in adults. QULIPTA is one of the world's first and only oral calcitonin gene-related peptide (CGRP) receptor antagonists (gepant) developed specifically for migraine prevention.
Thus, the market is expected to project growth over the forecast period. However, the growth of the migraine therapeutics market may be hampered due to a lack of proper diagnosis, undiagnosed cases, and drug side effects over the forecast period.
Migraine Therapeutics Market Trends
The Triptans Segment Is Expected to Grow at a Healthy CAGR Over the Forecast Period
Triptans are a newer class of drug that increases serotonin levels in the brain, reducing inflammation and constricting blood vessels, effectively ending a migraine. Triptans constrict blood vessels in the brain and block pain pathways. These ergotamines are more migraine-specific than previous ergotamines. Almotriptan (Axert), eletriptan (Relpax), frovatriptan (Frova), naratriptan (Amerge), rizatriptan (Maxalt, Maxalt-MLT), sumatriptan (Imitrex), sumatriptan and naproxen (Treximet), and zolmitriptan (Zomig) are some of the triptans available. Sumatriptan and naproxen sodium (Treximet), a single-tablet combination, are more effective than either medication alone in alleviating migraine symptoms.
Furthermore, as per the study titled "Comparison of New Pharmacologic Agents With Triptans for Treatment of Migraine" published in JAMA Network in October 2020, ditans and gepants were associated with less efficacy compared with triptans, whereas gepants were associated with fewer adverse events compared with triptans. Thus, due to the higher efficacy of the triptans, there is expected to be a rise in demand for them, thereby boosting the segment over the forecast period.
Moreover, the product launches and market approvals for drugs in the triptan class are also driving the growth of the market segment. For instance, in January 2020, Eli Lilly and Company launched the REYVOW (lasmiditan) C-V 50 mg and 100 mg tablets. It is an oral medication for the acute treatment of migraine with or without aura in adults.
Thus, owing to the aforementioned factors such as the higher efficacy of triptan and new product launches, the market segment is expected to boost the market over the forecast period.
North America is Expected to Hold a Significant Share Over the Forecast Period
North America is expected to dominate the overall market and is projected to maintain its dominance over the coming years. The high prevalence of migraine, the increasing adoption of novel therapeutics, and the large target population in the United States are the factors that contribute to the region's dominance.
According to the article titled "What is Migraine?" published in the JAMA Network in January 2022, in the United States, 17.1% of women and 5.6% of men reported having migraine symptoms. Thus, such a high prevalence is expected to boost the migraine therapeutics market in the United States.
Additionally, various strategic activities by key market players, such as product launch and approval, as well as mergers and acquisitions, will boost the market growth. For instance, in February 2020, Lundbeck received VYEPTI (eptinezumab-jjmr) approval by the United States Food and Drug Administration (FDA) for the preventive treatment of migraine in adults. Furthermore, in May 2021, Biohaven Pharmaceutical Holding Company Ltd. received United States Food and Drug Administration (FDA) approval for NURTEC ODT (rimegepant 75 mg) for the preventive treatment of migraine. NURTEC ODT is indicated for adult patients with episodic migraine, e.g., those who experience fewer than 15 headache days per month. Such approvals propel the growth of the studied market over the forecast period.
Thus, owing to the abovementioned factors, the North American region is expected to dominate the market over the forecast period.
Migraine Therapeutics Industry Overview
The migraine therapeutics market is moderately fragmented with the presence of a large number of local and international players. Key players are adopting different growth strategies to enhance their market presence, such as partnerships, agreements, collaborations, new product launches, geographical expansions, mergers, and acquisitions. Some of the key market players include Amgen, Teva Pharmaceutical, Eli Lilly and Company, and GlaxoSmithKline plc, among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Awareness about Migraine and its Treatment Options
- 4.2.2 Increasing R&D Expenditures Leading to High Pipeline Products
- 4.2.3 Increasing Prevalence of Migraines along with High Unmet Needs
- 4.3 Market Restraints
- 4.3.1 Adverse Effects of Drugs
- 4.3.2 Lack of Proper Diagnosis and Increasing Undiagnosed Cases
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD million)
- 5.1 By Therapeutics
- 5.1.1 Pain-relieving Medications
- 5.1.1.1 Analgesics
- 5.1.1.2 Triptans
- 5.1.1.3 Ergot Alkaloids
- 5.1.1.4 Others
- 5.1.2 Preventive Medications
- 5.1.2.1 Blood pressure-lowering Medications
- 5.1.2.2 Anticonvulsant Drugs
- 5.1.2.3 Calcitonin Gene-related Peptide (CGRP) Antagonists
- 5.1.2.4 Other Preventative Therapies
- 5.1.1 Pain-relieving Medications
- 5.2 By Route of Administration
- 5.2.1 Oral & Nasal
- 5.2.2 Injectables
- 5.3 Geography
- 5.3.1 North America
- 5.3.1.1 United States
- 5.3.1.2 Canada
- 5.3.1.3 Mexico
- 5.3.2 Europe
- 5.3.2.1 Germany
- 5.3.2.2 United Kingdom
- 5.3.2.3 France
- 5.3.2.4 Italy
- 5.3.2.5 Spain
- 5.3.2.6 Rest of Europe
- 5.3.3 Asia-Pacific
- 5.3.3.1 China
- 5.3.3.2 Japan
- 5.3.3.3 India
- 5.3.3.4 Australia
- 5.3.3.5 South Korea
- 5.3.3.6 Rest of Asia-Pacific
- 5.3.4 Middle-East and Africa
- 5.3.4.1 GCC
- 5.3.4.2 South Africa
- 5.3.4.3 Rest of Middle-East and Africa
- 5.3.5 South America
- 5.3.5.1 Brazil
- 5.3.5.2 Argentina
- 5.3.5.3 Rest of South America
- 5.3.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Amgen
- 6.1.2 Abbvie Inc.
- 6.1.3 AstraZeneca
- 6.1.4 Eli Lilly and Co.
- 6.1.5 GlaxoSmithKline
- 6.1.6 Merck & Co., Inc.
- 6.1.7 Pfizer
- 6.1.8 Bausch Health
- 6.1.9 Teva Pharmaceuticals
- 6.1.10 Novartis AG
- 6.1.11 Eisai Co., Ltd.,
- 6.1.12 Abbott Laboratories
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




