PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1437991

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1437991
Clinical Diagnostics - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029)
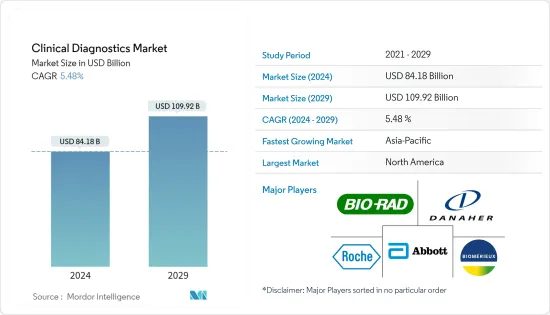
The Clinical Diagnostics Market size is estimated at USD 84.18 billion in 2024, and is expected to reach USD 109.92 billion by 2029, growing at a CAGR of 5.48% during the forecast period (2024-2029).
Owing to the COVID-19 pandemic, there had been an increase in lab testing, which saw demand grow even more rapidly to keep pace with the suspected cases of COVID-19. According to the Atlantic Monthly Group, there was a tremendous increase globally in COVID-19 tests, rising from 760,441 tests in September 2020 to 964,792 tests by October 2020. Thus, the rising number of tests owing to the constant upsurge in patients and funding by governments were the factors responsible to escalate the demand for COVID-19 test kits and drove the overall market growth exponentially. In addition, the demand for clinical diagnostics is expected to remain constant during the post-pandemic period due to the emergence of a mutant strain of the COVID-19 virus, thereby contributing to the growth of the market over the five years.
The market is expected to grow due to the increasing incidence of infectious as well as chronic diseases and the increasing adoption of automated platforms. According to the World Health Organization report in February 2022, each year, approximately 400,000 children and adolescents of 0-19 years old develop cancer. The same source also mentioned that the most common types of childhood cancers include leukemias, brain cancers, lymphomas, and solid tumors, such as neuroblastomas and Wilms tumors. The report Published by International Agency for Research on Cancer (IARC) in December 2020 that the global cancer burden has risen to 19.3 million cases. and thus, due to the high prevalence of cancer around the world, the demand for effective clinical diagnostics, thereby contributing to the growth of the market. Also, the rising infectious diseases are expected to contribute to the growth of the clinical diagnostics market. For instance, the report published by the World Health Organization in November 2021, reported that more than 1.0 million sexually transmitted infections are acquired globally and most of them are asymptomatic. It also reported that every year there are an estimated 374.0 million new infections with 1 out of 4 sexually transmitted infections: gonorrhea, chlamydia, trichomoniasis, and syphilis.
Owing to the increase in chronic diseases, the demand for healthcare systems is also increasing. Clinical diagnostics have, thus, proven to be beneficial in chronic disease conditions and are found to be valuable for disease prevention, detection, and management. Thus, the increasing incidence of chronic diseases is expected to propel the overall market further.
Clinical Diagnostics Market Trends
Lipid Panel Tests Segment is Expected to Register Good Growth in the Forecast Period
The lipid panel is a blood test that measures the lipids, fats, and fatty substances used as a source of energy by the body. The lipid panel includes tests for total cholesterol, HDL cholesterol, triglycerides, LDL cholesterol, cholesterol/HDL ratio, and non-HDL cholesterol.
A lipid panel measures the level of specific lipids in the blood to assess the risk of cardiovascular disease and is used in screening populations to identify subjects with a high risk of developing a cardiac event. The British Heart Foundation reported in January 2022 that the most common heart conditions affected globally were coronary (ischemic) heart disease (global prevalence estimated at 200 million), peripheral arterial (vascular) disease (110 million), stroke (100 million), and atrial fibrillation (60 million). Such a large prevalence globally is fueling the growth of the market. Furthermore, the Centers for Disease Control and Prevention (CDC) article updated in September 2021 reported that approximately 6.5 million people aged 40 and older in the United States have peripheral arterial disease. Additionally, the MDPI Journal research article published in September 2021 reported that the worldwide prevalence of peripheral arterial disease (PAD) is estimated to be 3%-12%, affecting nearly 27 million people in America and Europe. The same source also reported that in Europe, the prevalence of PAD is estimated at around 17.8% between the ages of 45 and 55. Thus, these studies showing the high burden of peripheral vascular diseases are expected to boost the growth of the market. Hence, with an increase in the prevalence and incidence rate of cardiovascular diseases, there is an increase in the requirement for lipid profile testing at an early stage, which is driving the lipid panel segment.
Also, the increasing number of product launches for lipid panel tests is also expected to contribute to the growth of the studied segment over the forecast period. For instance, in October 2022, Boston Heart Diagnostics announced the availability of LipoMap. This panel of 33 lipid, lipoprotein, and apolipoprotein tests is performed via high-resolution 600 MHz nuclear magnetic resonance and is one of the most comprehensive assessments of lipid metabolism commercially available.
Key companies are focusing on novel product developments and launches to leverage potential opportunities. Hence, the segment is estimated to grow over the forecast period due to the aforementioned factors.
North America is Expected to Dominate the Clinical Diagnostics Market Over the Forecast Period
The market for clinical diagnostics in North America is majorly driven by the increasing geriatric population, rising patient awareness about the value of laboratory tests, and the rising prevalence of infectious and chronic disease patients.
For instance, the data published by the American Cancer Society in 2022 mentioned that a total 1.9 million new cancer cases are expected to occur in the United States in 2022. Also, the Centers for Disease Control and Prevention in March 2021 published that more than 1 in 7, which is 15% of the United States adults are estimated to have chronic Kidney disease in 2021. such prevalence of chronic diseases is expected to drive the growth of the market.
Also, the rising funding activities to support the growth of clinical diagnostics are expected to contribute to the growth of the market in this region. For instance, in September 2020, the National Institutes of Health, US, announced its plan to give funding of USD 129.3 million to nine companies, including MatMaCorp, Maxim Biomedical Inc., MicroGEM International, etc., to scale-up manufacturing support for a new set of COVID-19 testing technologies as part of its Rapid Acceleration of Diagnostics (RADx) initiative.
This has led to a higher demand for better treatment with efficient management, further driving the market in the North America. Hence, the increasing prevalence of infectious and chronic diseases and the growing awareness of the value of laboratory tests are expected to fuel the market growth in the country.
Clinical Diagnostics Industry Overview
The market is highly competitive and consists of global and local players. The competitive landscape includes an analysis of a few international as well as local companies that hold market shares and are well known. The major key players in the market include Abbott Laboratories, Bio-Rad Laboratories Inc., Danaher Corporation, Becton, Dickinson and Company, Qiagen, and Roche Diagnostics.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Burden of Infectious and Chronic Diseases
- 4.2.2 Increasing Adoption of Automated Platforms
- 4.3 Market Restraints
- 4.3.1 Affordability for High-end Molecular Diagnostics
- 4.3.2 Limitations Associated with Reimbursement Scenario
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size in Value)
- 5.1 By Test
- 5.1.1 Lipid Panel
- 5.1.2 Liver Panel
- 5.1.3 Renal Panel
- 5.1.4 Complete Blood Count
- 5.1.5 Electrolyte Testing
- 5.1.6 Infectious Disease Testing
- 5.1.7 Other Tests
- 5.2 By Product
- 5.2.1 Instruments
- 5.2.2 Reagents
- 5.2.3 Other Products
- 5.3 By End User
- 5.3.1 Hospital Laboratory
- 5.3.2 Diagnostic Laboratory
- 5.3.3 Point-of-care Testing
- 5.3.4 Other End Users
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Abbott Laboratories
- 6.1.2 Becton, Dickinson and Company
- 6.1.3 BioMerieux
- 6.1.4 Bio-Rad Laboratories Inc.
- 6.1.5 Danaher Corporation
- 6.1.6 Siemens AG
- 6.1.7 Hologic Inc.
- 6.1.8 Qiagen NV
- 6.1.9 F. Hoffmann-La Roche AG
- 6.1.10 Thermo Fisher Scientific
- 6.1.11 Quest Diagnostics Inc.
- 6.1.12 Sysmex Corporation
- 6.1.13 Sonic Healthcare Ltd
- 6.1.14 Charles River Laboratories
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




