PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851888

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851888
Heparin - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
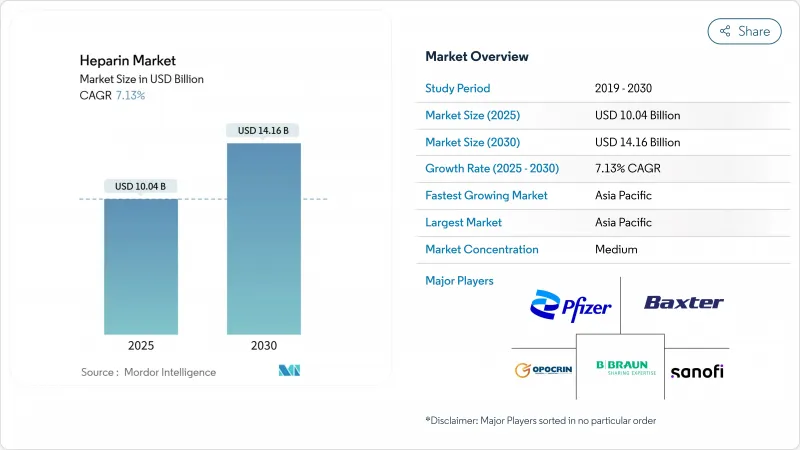
The heparin market size stands at USD 10.04 billion in 2025 and is projected to reach USD 14.16 billion by 2030, reflecting a 7.13% CAGR.
Rising surgical volumes, growth in dialysis procedures, and broader applications in oncology care and medical devices keep demand strong. Supply-chain diversification, particularly the move toward biosynthetic production, is emerging as a strategic priority as African Swine Fever-related shortages revealed the risks of heavy porcine dependence. Regulatory support for re-introducing bovine-sourced heparin and funding for bioengineered alternatives further strengthen the outlook. Competitive activity centers on portfolio expansion and geographic reach, while hospitals remain the largest buying segment.
Global Heparin Market Trends and Insights
Escalating Surgical Procedure Volumes and Dialysis Procedures
Cardiovascular and orthopedic surgeries continue to increase, and both settings rely on prophylactic anticoagulation, keeping heparin demand high. Dialysis sessions are also rising as end-stage renal disease prevalence climbs. Recent trials show that heparin-coated dialyzers plus intermittent saline flushing eliminated clotting events in high-bleeding-risk patients. Regional anticoagulation techniques now allow acid and heparin-free dialysis for select cases, yet overall procedure growth sustains volume through 2027.
Rapid Adoption of Low-Molecular-Weight Heparin and Biosynthetic Programs
Clinicians favor LMWH for its predictable pharmacokinetics and limited monitoring needs, supporting steady share gains. Parallel advances in bioengineered heparin show E. coli-based systems producing material chemically comparable to porcine products and convertible to LMWH. In 2025, the NHLBI funded a US USD 306,656 project to scale microbial production, underlining momentum for non-animal sources.
Supply-Chain Vulnerability due to Animal-Derived Raw Material
About 80% of active pharmaceutical ingredient volumes originate in China, leaving markets exposed to disease outbreaks and geopolitical actions. The 2019-2021 African Swine Fever crisis tightened supplies and lifted costs. In 2025, the FDA encouraged bovine-sourced heparin to diversify supply, citing improved purification that removes BSE prions.
Other drivers and restraints analyzed in the detailed report include:
- High Disease Burden and Aging Population
- Expanding Application in Medical Devices
- Risk and Adverse Bleeding Events
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Low-molecular-weight heparin held 63% of revenues in 2024, supported by once-daily dosing that suits outpatient use. The heparin market reports consistent switching from unfractionated heparin to LMWH in surgical prophylaxis and oncology settings. The segment's steady volume has prompted manufacturers to expand syringe formats for safer bedside administration.
Synthetic and biosynthetic heparin is the fastest-growing product line at 8.40% CAGR, reflecting investment in microbial and chemo-enzymatic pathways that bypass porcine inputs. As these alternatives reach scale, they may reshape overall heparin market share by the decade's end.
Porcine mucosa supplied 87.50% of global volume in 2024, anchoring production hubs in China's coastal provinces. The heparin market size for porcine source is sensitive to herd-health shocks and tariffs that raise cost for importing countries. Recombinant microbial sources, growing at 9.10% CAGR, attract private capital and public grants aimed at supply-chain resilience.
Bovine material is returning to regulated markets after updated FDA guidance demonstrated that modern purification removes BSE agents. Early adopters aim to smooth volatility in porcine supply while maintaining pharmacologic equivalence.
Heparin Market Report is Segmented Into by Product (Unfractionated Heparin, Low Molecular Weight Heparin and More), Source (Bovine and More), Dosage Form (Injectable Solution, Pre-Filled Syringe, and More), Route of Administration (Injectable and More), Application (Deep Vein Thrombosis and More), End User (Hospitals, Ambulatory Surgical Centers and More) and Geography. The Market and Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America recorded sizable demand in 2024, driven by high surgical procedure volumes and chronic dialysis populations. FDA encouragement for bovine sourcing seeks to stabilize supplies while tariffs on Chinese imports highlight procurement risks. Classification of new heparin test systems into Class II special controls exemplifies the tightening regulatory climate.
Asia-Pacific delivered 32.10% of global revenue and posted the fastest CAGR at 8.19%. China's processing base remains indispensable, though recent policy reviews urge stronger R&D and quality oversight to ensure enduring competitiveness. Emerging self-sufficiency programs in India and Southeast Asia aim to localize API production, potentially expanding regional heparin market supply.
Europe maintains a stable share as aging demographics and well-funded health systems support steady consumption. EMA conformity assessments for device-drug combinations enforce rigorous safety standards. Biosimilar pathways differ from US practice, influencing launch timelines and competitive intensity among low-molecular-weight brands.
- B. Braun
- Baxter
- Dr. Reddy's Laboratories
- Hebei Changshan Biochemical Pharmaceutical Co. Ltd.
- Leo Pharma
- Opocrin S.p.A.
- Pfizer
- Fresenius
- GLAND PHARMA
- Hikma Pharmaceuticals
- Techdow Pharma USA Inc.
- Viatris
- Abbott Laboratories
- Smiths Group
- Sanofi
- Shenzhen Hepalink Pharmaceutical Co. Ltd.
- Bioiberica
- Nanjing King-Friend Biochemical Pharmaceutical Co. Ltd.
- Cipla
- Emcure Pharmaceuticals Ltd
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Escalating Surgical Procedure Volumes and Dialysis Procedures
- 4.2.2 Rapid Adoption of Low-Molecular-Weight Heparin
- 4.2.3 High Burden of Targeted Diseases and Ageing Population
- 4.2.4 Expanding Application in Medical Devices
- 4.2.5 Increasing Application in Oncology Care
- 4.2.6 Escalating Use of Heparin
- 4.3 Market Restraints
- 4.3.1 Supply-Chain Vulnerability due to Dependence on Animal Derived Raw Material
- 4.3.2 Risk and Adverse Bleeding Events
- 4.3.3 Stringent Regulatory Requirements
- 4.3.4 Therapeutic Shift Toward Direct Oral Anticoagulants in Long-term Thromboprophylaxis
- 4.4 Value / Supply-Chain Analysis
- 4.5 Regulatory Outlook
- 4.6 Technological Outlook
- 4.7 Porter's Five Forces Analysis
- 4.7.1 Threat of New Entrants
- 4.7.2 Bargaining Power of Buyers
- 4.7.3 Bargaining Power of Suppliers
- 4.7.4 Threat of Substitute Products
- 4.7.5 Intensity of Competitive Rivalry
5 Market Size and Growth Forecasts (Value in USD)
- 5.1 By Product
- 5.1.1 Unfractionated Heparin
- 5.1.2 Low-Molecular-Weight Heparin
- 5.1.3 Ultra-Low-Molecular-Weight Heparin
- 5.1.4 Synthetic / Biosynthetic Heparin
- 5.2 By Source
- 5.2.1 Porcine
- 5.2.2 Bovine
- 5.2.3 Recombinant Microbial
- 5.3 By Dosage Form
- 5.3.1 Injectable Solution
- 5.3.2 Pre-filled Syringe
- 5.3.3 Topical Gel / Cream
- 5.3.4 Others
- 5.4 By Route of Administration
- 5.4.1 Intravenous
- 5.4.2 Sub-cutaneous
- 5.5 By Application
- 5.5.1 Deep Vein Thrombosis (DVT)
- 5.5.2 Atrial Fibrillation and Heart Attack
- 5.5.3 Coronary Artery Disease
- 5.5.4 Hemodialysis
- 5.5.5 Other Applications
- 5.6 By End User
- 5.6.1 Hospitals
- 5.6.2 Ambulatory Surgical Centers
- 5.6.3 Specialty Clinics
- 5.6.4 Home-care Settings
- 5.7 Geography
- 5.7.1 North America
- 5.7.1.1 United States
- 5.7.1.2 Canada
- 5.7.1.3 Mexico
- 5.7.2 Europe
- 5.7.2.1 Germany
- 5.7.2.2 United Kingdom
- 5.7.2.3 France
- 5.7.2.4 Italy
- 5.7.2.5 Spain
- 5.7.2.6 Rest of Europe
- 5.7.3 Asia-Pacific
- 5.7.3.1 China
- 5.7.3.2 Japan
- 5.7.3.3 India
- 5.7.3.4 South Korea
- 5.7.3.5 Australia
- 5.7.3.6 Rest of Asia-Pacific
- 5.7.4 Middle East and Africa
- 5.7.4.1 GCC
- 5.7.4.2 South Africa
- 5.7.4.3 Rest of Middle East and Africa
- 5.7.5 South America
- 5.7.5.1 Brazil
- 5.7.5.2 Argentina
- 5.7.5.3 Rest of South America
- 5.7.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Share Analysis
- 6.4 Company profiles (includes Global level Overview, Market level overview, Core Business Segments, Financials, Headcount, Key Information, Market Rank, Market Share, Products and Services, and analysis of Recent Developments)
- 6.4.1 B. Braun Melsungen AG
- 6.4.2 Baxter International Inc.
- 6.4.3 Dr. Reddy's Laboratories Ltd.
- 6.4.4 Hebei Changshan Biochemical Pharmaceutical Co. Ltd.
- 6.4.5 Leo Pharma A/S
- 6.4.6 Opocrin S.p.A.
- 6.4.7 Pfizer Inc.
- 6.4.8 Fresenius Kabi AG
- 6.4.9 Gland Pharma Ltd.
- 6.4.10 Hikma Pharmaceuticals PLC
- 6.4.11 Techdow Pharma USA Inc.
- 6.4.12 Viatris Inc.
- 6.4.13 Abbott
- 6.4.14 Smiths Medical (ICU Medical)
- 6.4.15 Sanofi S.A.
- 6.4.16 Shenzhen Hepalink Pharmaceutical Co. Ltd.
- 6.4.17 Bioiberica S.A.U.
- 6.4.18 Nanjing King-Friend Biochemical Pharmaceutical Co. Ltd.
- 6.4.19 Cipla
- 6.4.20 Emcure Pharmaceuticals Ltd
7 Market Opportunities and Future Outlook
- 7.1 White-space and Unmet-Need Assessment




