PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1852135

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1852135
Syndromic Multiplex Diagnostic - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The syndromic multiplex diagnostic market size reached USD 2.95 billion in 2025 and is forecast to climb to USD 3.86 billion by 2030, reflecting a 5.56% CAGR over the period.
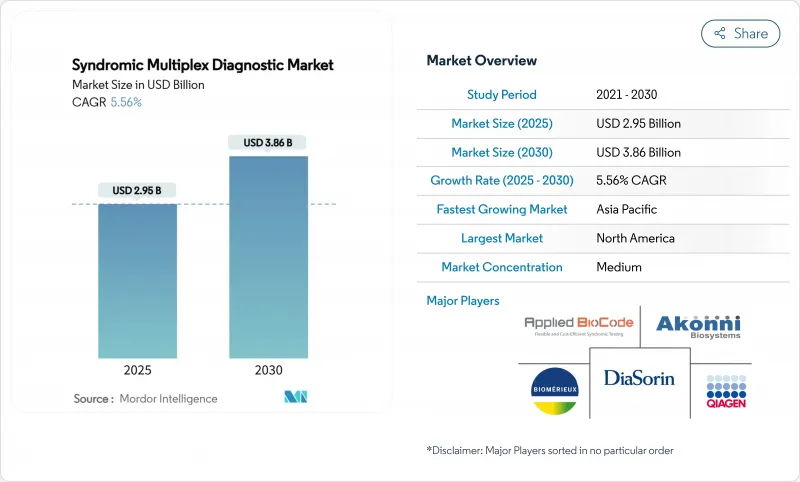
This expansion traces directly to a global shift toward precision testing that identifies multiple pathogens from a single specimen, shortening the path between specimen collection and targeted therapy. Respiratory test panels remain the primary revenue generator, fueled by post-pandemic hospital investments and physician familiarity with multiplex respiratory workflows. At the same time, neurology-focused panels outpace every other clinical category as clinicians seek faster answers for meningitis and encephalitis cases where delays translate into higher mortality. Technology adoption mirrors these clinical priorities: multiplex PCR continues to dominate, yet next-generation sequencing (NGS) platforms are posting the fastest unit growth as laboratories experiment with broader genomic profiling. Another catalyst is surging demand for near-patient testing; point-of-care systems that once delivered results in hours now return actionable answers in roughly 15 minutes, prompting payers to recognize the cost-of-care savings that flow from earlier intervention.
Global Syndromic Multiplex Diagnostic Market Trends and Insights
Rising Prevalence of Infectious Diseases
Resurgent viral and bacterial threats elevate demand for broad respiratory, gastrointestinal, and febrile-illness panels that can distinguish co-infections in a single run. WHO's 2024 global health spending study confirmed pandemic-era budget reallocations away from prevention and toward acute care, leaving diagnostic gaps that multiplex assays now fill. Anti-microbial resistance compounds the need for precise identification because empiric therapy risks treatment failure. The United States FDA's emergency-use authorizations for emerging agents such as monkeypox in 2024 underscored regulators' desire for deployable panels that laboratories can reconfigure quickly. This flexibility attracts public-health buyers in Africa and Southeast Asia, where reference labs often sit far from outbreak zones. Hospitals in Western Europe, meanwhile, are integrating resistance-marker detection into routine sepsis work-ups to reduce broad-spectrum antibiotic exposure.
Growing Adoption of Molecular Diagnostic Technologies
Healthcare administrators increasingly weigh total-cost-of-care savings rather than per-test price. Multiplex PCR cuts average hospital stays by one day in acute respiratory cases, offsetting higher reagent costs. QIAGEN received multiple FDA clearances for its QIAstat-Dx respiratory and CNS panels during 2024, illustrating how regulators are streamlining pathways for syndromic submissions. Digital PCR and isothermal methods have matured; some platforms now detect variants at 0.01% allele frequency, enabling oncology and transplant monitoring as additional revenue streams. CLIA waiver status has widened access in U.S. outpatient clinics, strengthening the syndromic multiplex diagnostic market's penetration outside tertiary centers.
High Cost of Syndromic Test Panels
Pricing remains the chief obstacle to universal adoption. A respiratory panel in India can cost as much as an entire course of inpatient antibiotics, forcing provincial hospitals to ration testing. Payers in the United States ask manufacturers to submit clinical-utility dossiers under the MolDX program to justify coverage beyond algorithmic sensitivity claims. Panel size inflation further strains budgets; a 30-target assay may exceed reimbursement caps even when only a subset of targets is clinically relevant. Stakeholders therefore explore risk-sharing models that link payment to reduced hospitalization or antibiotic use, though such agreements require longitudinal outcomes data that few suppliers collect today.
Other drivers and restraints analyzed in the detailed report include:
- Expanding Point-of-Care Testing Infrastructure
- Technological Advancements in Multiplex PCR and NGS Platforms
- Limited Skilled Workforce in Molecular Diagnostics
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Respiratory assays generated 42.45% of 2024 revenue for the syndromic multiplex diagnostic market, highlighting entrenched reimbursement and clinician familiarity. Conversely, central nervous system panels are forecast to post a 7.65% CAGR through 2030 as neurologists embrace rapid testing that guides early antiviral or antibacterial therapy. This rising demand could lift the CNS segment's share of the syndromic multiplex diagnostic market size from the current mid-teens toward one-quarter by decade's end. Clinicians note that culture-first strategies miss pathogens in up to 50% of meningitis cases, making multiplex molecular results indispensable for pediatric and transplant populations. Panel commoditization pressures are more pronounced in respiratory testing, prompting vendors to differentiate via faster run times and connectivity features rather than pure analytic accuracy. Karius earned a Breakthrough Device nod for a lung-infection assay that combines metagenomic sequencing with host-response markers, signaling a new frontier in respiratory care.
Meanwhile, gastrointestinal and urogenital panels continue their steady climb, although their contribution to overall revenue trails respiratory and CNS testing. Rising antibiotic resistance in enteric pathogens and the need for stewardship programs stimulate orders for GI panels among tertiary centers in South and Southeast Asia. Urinary-tract and sexually-transmitted-infection panels benefit from CLIA-waived over-the-counter approvals, positioning community clinics as an incremental volume engine. FDA's creation of a separate device class for acute febrile illness panels opens doors for multi-syndrome cartridges that span respiratory and systemic infections, potentially blurring current segmentation demarcations.
Multiplex PCR still delivered 58.45% of 2024 revenue, securing the largest slice of the syndromic multiplex diagnostic market share. Its cost-per-sample and turnkey workflows keep it embedded in hospital labs. Yet NGS is tracking an 8.12% CAGR and could absorb a meaningful fraction of incremental spend as sequencing costs drop. Laboratories value NGS for unbiased detection across bacteria, fungi, viruses, and parasites-especially in chronic or immunocompromised cohorts where odd pathogens thrive. The syndromic multiplex diagnostic market size attached to NGS applications could approach USD 1 billion by 2030 if reimbursement pathways align with clinical need.
Microarrays are gradually ceding ground; their limited dynamic range and cumbersome workflows deter new installations. Digital PCR holds a niche in monitoring low-frequency resistance mutations and minimal residual disease in oncology, but its capital intensity limits penetration. Suppliers increasingly bundle multiple chemistries into a single chassis; DiaSorin's Liaison Plex mixes PCR and bead-based immunodetection in a modular system cleared by FDA in 2024, hinting at hybrid future architectures.
The Syndromic Multiplex Diagnostic Market Report is Segmented by Type of Syndrome (Respiratory, Gastrointestinal, Central Nervous System, and CUTI & STDs), Technology Platform (Multiplex PCR, and More), Panel Size (<=10 Targets, and More), End-User (Hospitals, and More), and Geography (North America, Europe, Asia-Pacific, Middle East & Africa, South America). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America retained 41.43% of 2024 revenue owing to established reimbursement, electronic medical-record integration, and a mature outpatient POC network. The syndromic multiplex diagnostic market size for the region could reach USD 1.6 billion by 2030 under current policy momentum. CMS reimbursement boosts adoption of respiratory and sepsis panels, while venture funding supports start-ups targeting over-the-counter molecular tests. Hospital groups increasingly deploy centralized-data analytics to correlate panel results with antimicrobial stewardship dashboards, reinforcing adoption.
Europe follows with steady but slower expansion as IVDR compliance demands continuous post-market surveillance data. Public health agencies subsidize multiplex installations in border countries to detect migrant-related outbreaks rapidly. Regional growth leans on mid-throughput laboratory systems rather than POC devices, yet CLIA-equivalent waiver concepts under discussion could spur pharmacy adoption in Germany and France once implemented.
Asia-Pacific posts the strongest trajectory at a 6.43% CAGR. China's 8.5 trillion-yuan health outlay in 2022 (7.05% of GDP) and India's projected 10-12% diagnostics growth between FY 2024 and FY 2028 are funneling capital toward syndromic platforms that tackle dengue, scrub typhus, and multi-drug-resistant tuberculosis. Public-private partnerships supply district hospitals with cartridge-based PCR units backed by cloud connectivity. Japan and South Korea continue upgrading hospital labs with NGS to support precision medicine, indirectly broadening microbial sequencing capacity.
South America and the Middle East & Africa trail in revenue but demonstrate meaningful growth pockets. Brazil invests in respiratory multiplex testing for regional influenza surveillance, while Saudi Arabia pilots CNS panels in tertiary centers managing high transplant volumes. In Sub-Saharan Africa, AFENET's CoLTeP initiative helps laboratories secure accreditation, unlocking donor funds for multiplex installations that handle HIV-adjacent opportunistic infections.
- bioMerieux
- Danaher (Cepheid)
- F. Hoffmann-La Roche (GenMark)
- Abbott Laboratories
- QIAGEN
- DiaSorin S.p.A (Luminex)
- Thermo Fisher Scientific
- Seegene
- Beckton Dickinson
- Hologic Inc. (Mobidiag)
- Randox Laboratories
- Genetic Signatures
- Meridian Bioscience
- Bosch Healthcare Solutions
- Biocartis
- AusDiagnostics
- Accelerate Diagnostics
- OpGen (Curetis)
- Akonni Biosystems, Inc.
- QuantuMDx
- Applied BioCode
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rising Prevalence of Infectious Diseases
- 4.2.2 Growing Adoption of Molecular Diagnostic Technologies
- 4.2.3 Expanding Point-of-Care Testing Infrastructure
- 4.2.4 Favorable Reimbursement Policies for Syndromic Panels
- 4.2.5 Increasing Healthcare Expenditure in Emerging Economies
- 4.2.6 Technological Advancements in Multiplex PCR and NGS Platforms
- 4.3 Market Restraints
- 4.3.1 High Cost Of Syndromic Test Panels
- 4.3.2 Limited Skilled Workforce in Molecular Diagnostics
- 4.3.3 Stringent Regulatory Approval Processes
- 4.3.4 Inadequate Laboratory Information System Integration
- 4.4 Regulatory Landscape
- 4.5 Porter's Five Forces Analysis
- 4.5.1 Threat of New Entrants
- 4.5.2 Bargaining Power of Buyers
- 4.5.3 Bargaining Power of Suppliers
- 4.5.4 Threat of Substitutes
- 4.5.5 Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Type of Syndrome
- 5.1.1 Respiratory
- 5.1.2 Gastrointestinal
- 5.1.3 Central Nervous System
- 5.1.4 cUTI & STDs
- 5.2 By Technology Platform
- 5.2.1 Multiplex PCR
- 5.2.2 Microarray-Based
- 5.2.3 Isothermal Amplification
- 5.2.4 NGS-Based
- 5.3 By Panel Size
- 5.3.1 <=10 Targets
- 5.3.2 11-20 Targets
- 5.3.3 >20 Targets
- 5.4 By End-User
- 5.4.1 Hospitals
- 5.4.2 Diagnostic Laboratories
- 5.4.3 Point-Of-Care / Retail Clinics
- 5.5 Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East & Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East & Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Business Segments, Financials, Headcount, Key Information, Market Rank, Market Share, Products and Services, and analysis of Recent Developments)
- 6.3.1 bioMerieux
- 6.3.2 Danaher (Cepheid)
- 6.3.3 F. Hoffmann-La Roche (GenMark)
- 6.3.4 Abbott
- 6.3.5 Qiagen
- 6.3.6 DiaSorin S.p.A (Luminex)
- 6.3.7 Thermo Fisher Scientific
- 6.3.8 Seegene Inc.
- 6.3.9 Becton, Dickinson and Company
- 6.3.10 Hologic Inc. (Mobidiag)
- 6.3.11 Randox Laboratories
- 6.3.12 Genetic Signatures
- 6.3.13 Meridian Bioscience
- 6.3.14 Bosch Healthcare Solutions
- 6.3.15 Biocartis
- 6.3.16 AusDiagnostics
- 6.3.17 Accelerate Diagnostics
- 6.3.18 OpGen (Curetis)
- 6.3.19 Akonni Biosystems, Inc.
- 6.3.20 QuantuMDx
- 6.3.21 Applied BioCode
7 Market Opportunities & Future Outlook
- 7.1 White-Space & Unmet-Need Assessment




