PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1437924

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1437924
Global Medical Device Testing and Certification - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029)
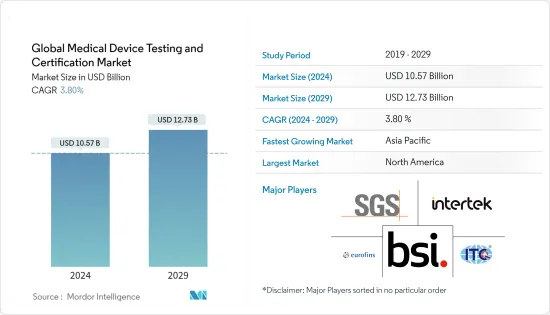
The Global Medical Device Testing and Certification Market size is estimated at USD 10.57 billion in 2024, and is expected to reach USD 12.73 billion by 2029, growing at a CAGR of 3.80% during the forecast period (2024-2029).
During the COVID-19 outbreak in 2020, several measures were taken to prevent the transmission of diseases, such as lockdown measurements and restricting import-export activities between the countries, which disrupted the supply chain, thus, negatively impacting the overall market studied.
Moreover, the disruption caused due to COVID-19 in international supply chains has led to shortages of critical medical devices across the world. Therefore, many countries have taken definite measures to ease the shortages by importing equipment, such as domestic manufacturing of medical devices. Additionally, domestic manufacturing of essential medical devices is expected overcome trade barriers, at the same time, ensure product quality and market stability.
As per British Standards Institution (BSI), in February 2020, by taking into consideration the effects of the COVID-19 pandemic, the company reviewed processes and planned a program to minimize the risk to British Standards Institution (BSI) clients and colleagues while maintaining accreditation requirements and mitigating potential global trade risks.
The medical devices are subjected to comply with strict regulatory protocols, as it is vital to ensure the efficacy and safety of medical devices. Therefore, it is compulsory for every device to comply with national and international standards before entering a market. The standard guidelines of medical devices vary from country to country, and it is mandatory for every manufacturer to follow these guidelines for marketing or selling their products in a country. For instance, the United States follows Food and Drug Administration (FDA) guidelines, Europe considers Conformite Europeenne (CE) approval, Canada needs Health Canada Registration, and India requires approval from Central Drugs Standard Control Organisation (CDSCO). This diverse range of regulatory landscapes drives the testing and certification market.
Since regulations are different in every country, it is crucial for each medical device manufacturer to register or receive regulatory guidelines of that specific country, which, in turn, indicates the need for authorized third parties to register their devices. National regulatory authorities of every country prefer that the manufacturers selling their products in that particular country should comply with standard guidelines and get it checked by a third-party certification system.
This may result in propelling the testing and certification market, as well as increase easy market access. The other factors, such as the increasing need for validation and verification (V&V) for medical devices, are driving the medical device testing and certification market. However, a factor such as diversity in regulation is expected to impede market growth over the forecast period.
Medical Device Testing Market Trends
Testing Services Segment is Expected to Witness Rapid Growth During the Forecast Period
Globally, medical devices are regulated by various regulatory authorities and compliances. This is mainly because the end-users of these devices expect outstanding performance, effectiveness, and safety from these medical devices. Therefore, it is mandatory for the manufacturers to properly define and implement a medical device testing strategy, which makes the device effective and production becomes easier due to the confirmation of quality.
In April 2020, due to the COVID-19 pandemic, the European Commission (EC) adopted a proposal to postpone the application date of the Medical Device Regulation (MDR) for one year because the COVID-19 pandemic increased demand for certain medical devices, which were crucial to avoid risks or difficulties of potential shortages of such devices. Moreover, the COVID-19 pandemic has delayed clinical trials and disrupted processes for medical devices.
Some of the medical devices experienced a sudden surge in demand during the COVID-19 pandemic 2020. For instance, ventilators were in high demand for COVID-19 patients, as they are an important tool in hospitals that can keep the patients in critical conditions alive. For instance, in March 2020, Medtronic PLC announced that it had increased production by more than 40% to date and was on track to more than double its capacity to manufacture and supply ventilators in response to the urgent needs of patients and healthcare systems across the world confronting COVID-19.
In June 2020, Intertek Group PLC announced the expansion of personal protective equipment services to include testing of N95 respirators to requirements set by the National Institute for Occupational Safety and Health (NIOSH). With this expansion, the company also expanded upon its solutions and resources to support customers and the global community during the COVID-19 pandemic.
An effective medical device testing strategy needs several sets of test requirements. The sets of requirements are required to smoothen test implementation as tests are carried out continuously at different stages of the complete manufacturing process, from component selection to a final assembly of a medical device. Each stage has different requirements and different parameters to be satisfied. Thus, increasing medical devices may also increase these testing services, which is expected to augment the overall growth of the market.
North America Dominates the Market and is Expected to do the Same over the Forecast Period
Some of the factors driving the market growth in the North American region include increased focus on the quality of the medical devices and the presence of a large number of companies that serve the medical device industry, along with the presence of well-developed healthcare and the presence of top multinational medical device companies.
According to a research article by A. Chandimal Nicholas published in May 2020, in Canada, during the COVID-19 pandemic 2020, the Minister of Health signed the Interim Order Respecting the Importation and Sale of Medical Devices for Use in relation to COVID-19, which allowed expedited access to COVID-19 medical devices for use by healthcare providers. Moreover, as per Health Canada, the Interim Order helped for quick approval of the importation and sale of COVID-19 medical devices.
Additionally, during the COVID-19 pandemic 2020, in the United States, the Food and Drug Administration (FDA) issued Emergency Use Authorizations (EUA) for medical devices to diagnose COVID-19 and personal protective equipment needed to protect healthcare providers interacting with patients.
In the United States, medical devices are managed by the Food and Drug Administration (FDA) to guarantee the safety and effectiveness of devices. The Center for Devices and Radiological Health (CDRH) is a Food and Drug Administration (FDA) segment. Class II devices require remarkable controls for 'labeling, guidance, tracking, plan, performance standards, and post-market observation,' and most require premarket notification 510(k) to appraise substantial equivalence to a lawfully marketed device.
According to the Center for Medicare & Medicaid Services, the United States healthcare spending grew by 4.6% in 2018, reaching USD 3.6 trillion or USD 11,172 per person. As a share of the nation's Gross Domestic Product, health spending accounted for 17.7%. Moreover, with increasing approval of medical devices in the region, rising demand for medical device testing services may boost the market growth. Thus, considering the above-mentioned factors, it is expected to fuel the market growth in the North American region over the forecast period.
Medical Device Testing Industry Overview
The medical device testing and certification market is highly consolidated, and few companies provide testing and certification services. It has been observed that with the growing medical device market, more companies are expected to enter the market in the future. Substantial market share may be gained by the small to mid-sized companies in the coming years. Some market players include BSI Group, Intertek Group PLC, Institute for Testing and Certification Inc., Eurofins Scientific, and SGS SA, among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Need of Validation and Verification (V&V) for Medical Devices
- 4.2.2 Compliance of Standards
- 4.3 Market Restraints
- 4.3.1 Diversity in Regulation
- 4.4 Industry Attractiveness - Porter's Five Forces Analysis
- 4.4.1 Bargaining Power of Buyers/Consumers
- 4.4.2 Bargaining Power of Suppliers
- 4.4.3 Threat of New Entrants
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION
- 5.1 By Service Type
- 5.1.1 Testing Services
- 5.1.2 Inspection Services
- 5.1.3 Certification Services
- 5.1.4 Other Services
- 5.2 By Sourcing Type
- 5.2.1 In-house
- 5.2.2 Outsourced
- 5.3 By Device Class
- 5.3.1 Class I
- 5.3.2 Class II
- 5.3.3 Class III
- 5.4 By Technology
- 5.4.1 Active Implant Medical Device
- 5.4.2 Active Medical Device
- 5.4.3 Non-active Medical Device
- 5.4.4 In Vitro Diagnostic Medical Device
- 5.4.5 Ophthalmic Medical Device
- 5.4.6 Orthopedic and Dental Medical Device
- 5.4.7 Other Technologies
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East & Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East & Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 BSI Group
- 6.1.2 Dekra Testing and Certification GmbH
- 6.1.3 Eurofins Scientific
- 6.1.4 Institute for testing and Certification Inc.
- 6.1.5 Intertek Group PLC
- 6.1.6 SGS SA
- 6.1.7 TUV Rheinland
- 6.1.8 UL LLC
- 6.1.9 Bureau Veritas
- 6.1.10 Element Materials Technology
- 6.1.11 Avomeen
- 6.1.12 Gateway Analytical LLC
- 6.1.13 Medistri SA
- 6.1.14 Pace Analytical Services LLC
- 6.1.15 WuXi AppTec
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




