Need help finding what you are looking for?
Contact Us
PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1405688

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1405688
Coronary Guidewires - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts 2024 - 2029
PUBLISHED:
PAGES: 111 Pages
DELIVERY TIME: 2-3 business days
SELECT AN OPTION
The coronary guidewires market is expected to register a CAGR of 5.1% over the forecast period.
Key Highlights
- The COVID-19 pandemic had a significant impact on the market. The market witnessed a slight negative impact during the initial days of the pandemic as the number of patients attending emergency departments witnessed a decrease during the pandemic. For instance, as per the NCBI article published in May 2022, there was a substantial decrease in the number of patients attending emergency departments with acute coronary syndromes (ACS), and there has been a considerable rise in early mortality or complications during the COVID-19 pandemic. The immediate percutaneous coronary intervention was performed less frequently on ACS patients in the COVID-19 group compared to the pre-COVID-19 group. However, during the pandemic, cardiovascular complications increased among COVID-19 patients, which positively impacted the market during later times. For instance, according to an NCBI article published in June 2022, patients with COVID-19 commonly have manifestations of heart disease, including signs of myocardial injury. The main causes of myocardial injury in patients with COVID-19 include hypoxic injury, stress cardiomyopathy, ischemic injury caused by cardiac microvascular dysfunction, small vessel cardiac vasculitis, endothelins, or epicardial coronary artery disease, and many more. All these disorders and related health issues increase the cardiac diseases in COVID patients. Thus, initially, the market witnessed a slight drop; however, with the rising patient inflow and resumption of cardiac procedures, the market gained traction and is expected to maintain an upward trend over the forecast period.
- The increasing prevalence of cardiovascular diseases and the rise in several interventional radiology procedures are the major drivers for the market. For instance, according to the data published by the British Heart Foundation in August 2022, in the United Kingdom, over 7.6 million people lived with heart or circulatory disease in 2021. According to an NCBI research study published in August 2022, cardiovascular diseases were highly prevalent in the rural areas of South Asia, with an incidence of 5.41 per 1,000 person-years as compared to 4.73 per 1,000 person-years in urban areas, and the incidence was highest among the males as compared to females. Thus, the increasing prevalence of cardiovascular diseases may propel the demand for coronary guidewires, owing to which the coronary guidewires market is anticipated to witness considerable growth over the forecast period,
- The increasing preference towards minimally invasive treatment and favorable initiatives by the key players are also driving the market growth. For instance, according to the NCBI research article published in February 2022, in the studied population, about 57.0% of the patients who preferred minimally invasive treatment made their decision under the influence of the healthcare professionals as they recommended minimally invasive treatment and reported less perceived disadvantages following their treatment. Such studies depict the higher acceptance of healthcare professionals and patients of minimally invasive procedures over traditional procedures. Similarly, the NCBI study published in June 2022 reported that in Germany, 2,834 aortic valve procedures, accounting for nearly 36.8% of the total aortic valve procedures, and 3,369 mitral valve operations, accounting for nearly 55.7% of the total mitral valve operations were performed via minimally invasive access in 2021. Therefore, increasing the adoption of minimally invasive procedures may positively impact the market growth.
- However, the high cost of minimally invasive surgeries and complications associated with guidewire use are expected to restrain the growth of the market over the forecast period. In January 2023, Deepwise Technology Co. Ltd.'s artificial intelligence (AI) device mammography screening software was approved in China. The company claims this is the first and only AI medical device approved for breast cancer in China.
- In September 2022, Shenzhen Anke's new generation of Xinxin digital mammary gland machine was approved for listing in China. The product has digital mammography, digital mammography tomography, and digital mammography breast tomosynthesis 2D imaging function.
Coronary Guidewires Market Trends
Stainless Steel Segment is Expected to Witness Considerable Growth Over the Forecast Period
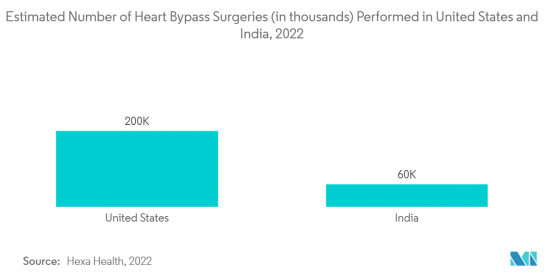
- Stainless steel coronary guidewires are specialized wires used in angioplasty procedures to introduce and position balloon catheters and other devices within the coronary system. They are non-magnetic, non-staining, and corrosion-resistant, with antibacterial properties. Thus, the beneficial factors of stainless steel coronary guidewires are expected to promote usage, increase the demand for various surgical procedures, and boost the growth of the segment studied. For instance, according to the research article published by Hexa Health in October 2022, around 4,00,000 heart bypass surgeries are performed every year globally. Such an increasing number of cardiovascular surgeries is expected to increase the demand for stainless steel coronary guidewires as it is one of the essential requirements, thereby propelling the segment's growth.
- The product approvals and technological advancements related to products are also propelling segment growth. For instance, as per an NCBI article published in 2022, many researchers and new startups have come up with innovations of coronary guidewires with antibacterial properties to avoid post-operative infections and complications. Such innovations are expected to accelerate the demand for stainless steel coronary guidewires and boost the segment's growth. The increasing product approvals are expected to contribute to the growth of the segment studied over the forecast period. For instance, in June 2022, Cardio Flow Inc. received United States Food and Drug Administration (FDA) clearance for its FreedomFlow Peripheral Guidewire. The FreedomFlow guidewire is a stainless steel core-to-tip design with a fixed distal-spring roll. Therefore, owing to increasing product approvals and innovations and cardiovascular procedures are expected to drive the segment's growth in the market over the forecast period.
North America is Expected to Witness Significant Growth Over the Forecast Period
- North America is expected to hold a major market share in the global coronary guidewires market due to the increasing prevalence of cardiovascular diseases, the rise in the number of interventional diagnostic procedures, and the growing demand for minimally invasive surgeries in this region. Furthermore, according to the data published in February 2022 by the Heart and Stroke Foundation Canada, in Canada, more than 750,000 people are living with heart failure, and more than 100,000 people are diagnosed with this incurable condition each year. Furthermore, many approvals for coronary guidewires in the region are expected to drive market growth.
- For instance, in April 2022, OpSens Inc. received Health Canada approval for the SavvyWire, its new guidewire for transcatheter aortic valve replacement procedures, or TAVR. In May 2022, Medtronic received FDA approval for its IN.PACT 018 Paclitaxel-Coated Percutaneous Transluminal Angioplasty (PTA) Balloon Catheter to treat patients with peripheral arterial disease (PAD) in the superficial femoral and popliteal arteries. Thus, owing to the above-mentioned factors, considerable market growth is expected in the North American region over the forecast period.
- Key product launches, high concentration of market players or manufacturers' presence, acquisition and & partnerships among major players, and increasing prevalence of cardiovascular diseases in the United States are some of the factors driving the growth of the coronary guidewires market in the country. For instance, according to the October 2022 update of the CDC, more than 805 thousand people in the United States have a heart attack every year, and an estimated 20.1 million adults in the country had coronary artery disease in 2021. In addition, in February 2022, Teleflex Incorporated announced that the USFDA cleared an expanded indication for its specialty catheters and coronary guidewires for use in crossing chronic total occlusion percutaneous coronary interventions (CTO PCI). Therefore, factors such as the increasing prevalence of cardiovascular diseases and product launches are expected to drive the market's growth in the United States over the forecast period.
Coronary Guidewires Industry Overview
The coronary guidewires market is consolidated in nature and consists of a few major players. In terms of market share, a few major players dominate the market. Some companies dominating the market are Abbott Laboratories, Terumo Medical Corporation, Boston Scientific Corporation, Cardinal Health Inc., Merit Medical Systems, Integer Holdings Corporation, BIOTRONIK SE & Co. KG, Medtronic PLC, JOTEC GmbH, and QXMedical LLC, among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
Product Code: 67728
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definitions
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Prevalence of Cardiovascular Diseases
- 4.2.2 Rise in Number of Interventional Radiology Procedures
- 4.3 Market Restraints
- 4.3.1 High Cost of Minimally Invasive Surgeries
- 4.3.2 Complications Associated With Guidewire Use
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD)
- 5.1 By Material
- 5.1.1 Nitinol
- 5.1.2 Stainless Steel
- 5.1.3 Other Materials
- 5.2 By Coating
- 5.2.1 Coated
- 5.2.1.1 Hydrophilic
- 5.2.1.2 Hydrophobic
- 5.2.2 Non Coated
- 5.2.1 Coated
- 5.3 By Geography
- 5.3.1 North America
- 5.3.1.1 United States
- 5.3.1.2 Canada
- 5.3.1.3 Mexico
- 5.3.2 Europe
- 5.3.2.1 Germany
- 5.3.2.2 United Kingdom
- 5.3.2.3 France
- 5.3.2.4 Italy
- 5.3.2.5 Spain
- 5.3.2.6 Rest of Europe
- 5.3.3 Asia-Pacific
- 5.3.3.1 China
- 5.3.3.2 Japan
- 5.3.3.3 India
- 5.3.3.4 Australia
- 5.3.3.5 South Korea
- 5.3.3.6 Rest of Asia-Pacific
- 5.3.4 Middle East and Africa
- 5.3.4.1 GCC
- 5.3.4.2 South Africa
- 5.3.4.3 Rest of Middle East and Africa
- 5.3.5 South America
- 5.3.5.1 Brazil
- 5.3.5.2 Argentina
- 5.3.5.3 Rest of South America
- 5.3.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Abbott Laboratories
- 6.1.2 Terumo Medical Corporation
- 6.1.3 Boston Scientific Corporation
- 6.1.4 Cardinal Health Inc.
- 6.1.5 Merit Medical Systems
- 6.1.6 Integer Holdings Corporation
- 6.1.7 BIOTRONIK SE & Co. KG
- 6.1.8 Medtronic PLC
- 6.1.9 JOTEC GmbH
- 6.1.10 QXMedical LLC
7 MARKET OPPORTUNITIES AND FUTURE TRENDS
Have a question?


SELECT AN OPTION
Have a question?


Questions? Please give us a call or visit the contact form.
