PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1689779

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1689779
PD-1 And PD-L1 Inhibitors - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
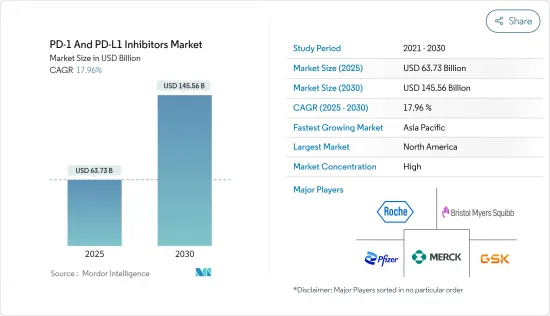
The PD-1 And PD-L1 Inhibitors Market size is estimated at USD 63.73 billion in 2025, and is expected to reach USD 145.56 billion by 2030, at a CAGR of 17.96% during the forecast period (2025-2030).
Several factors are projected to drive the growth of the PD-1 and PD-L1 inhibitors market. These include increased investments in research and development (R&D), a rise in clinical trials by bio-pharmaceutical companies, faster approvals from regulatory authorities, and a growing global cancer burden.
Numerous companies and research institutions are intensifying their efforts to develop new PD-1 inhibitors for various cancers. This strengthened pipeline, along with an increase in approvals, is expected to fuel market growth. For instance, in June 2023, Cancer Focus Fund, LP, in partnership with The University of Texas MD Anderson Cancer Center, announced a USD 4.5 million investment. This funding was allocated for the Phase 1a/1b clinical trial of ImmunoGenesis' lead candidate, IMGS-001. IMGS-001, a dual-specific PD-L1/PD-L2 antibody developed by ImmunoGenesis, targets immune-excluded tumors resistant to current immunotherapies. The investment specifically supports the multi-site Phase 1a/1b clinical trial of IMGS-001 at MD Anderson. Such an increase in trials underscores the heightened R&D activities in the PD-1 and PD-L1 inhibitors market, indicating a robust therapy pipeline and encouraging further exploration of innovative therapeutic approaches, thus driving market growth.
Over the forecast period, the market is expected to benefit from a significant rise in global cancer cases. For instance, data from the American Cancer Society (ACS) projects that by January 2024, the U.S. cancer patient count will reach 2.0 million, up from 1.95 million in 2023. PD-1, when binding to proteins like PD-L1, inhibits T cells from attacking other cells, including cancerous ones. Thus, the rising cancer cases are anticipated to increase the demand for PD-1 and PD-L1 inhibitors, known for their promising results, thus driving market growth over the forecast period.
Additionally, product approvals and active clinical trials by key players are expected to further stimulate the market. For instance, in February 2023, BeiGene's PD-1 inhibitor, tislelizumab, received approval from China's National Medical Products Administration (NMPA). This approval was for tislelizumab's use in combination with fluoropyrimidine and platinum chemotherapy, marking it as a first-line treatment for patients with high PD-L1 expression diagnosed with locally advanced unresectable or metastatic gastrointestinal adenocarcinoma.
In summary, driven by intensified R&D activities, a rising cancer incidence, and product approvals, the market is poised for growth. However, challenges such as the high costs and associated complications of oncology treatments, uncertainties in the regulatory landscape, and the steep expenses of clinical trials could temper this growth trajectory.
PD-1 And PD-L1 Inhibitors Market Trends
PD-1 Inhibitors Segment is Expected to Witness Significant Growth Over the Forecast Period
PD-1 (programmed cell death protein 1) inhibitors, a class of immunotherapy drugs, are significantly impacting the cancer treatment market. These agents have demonstrated substantial clinical benefits across various cancer types and have transformed the treatment landscape, particularly for patients with advanced or metastatic diseases, often outperforming traditional chemotherapy.
PD-1 inhibitors offer numerous advantages: they enhance the immune response, improve survival rates, reduce toxicity, and provide the potential for prolonged responses. Additionally, these inhibitors can be effectively combined with other cancer treatments, including chemotherapy, radiation, targeted therapies, and other immunotherapies. Such combinations have frequently shown synergistic effects, improving response rates and patient outcomes.
Ongoing research is exploring the broader potential of PD-1 inhibitors across various diseases. For instance, in February 2024, a collaborative effort led by researchers from the University of Hong Kong's Department of Microbiology and other esteemed institutions revealed that PD-1-boosted DNA vaccinations could sustain virus-specific CD8+ T cell immunity in an AIDS monkey model.
Similarly, an August 2023 study published by ASH publications highlighted an open-label phase 1/2 trial. This trial examined the combination of Favezelimab (anti-LAG-3) and Pembrolizumab in patients with relapsed Hodgkin lymphoma (cHL) post anti-PD-1 treatment. The findings indicated that heavily pretreated patients, whose cHL progressed after anti-PD-1 therapy, exhibited significant antitumor activity with manageable safety. Furthermore, research from the National Institute of Health in August 2022 emphasized the benefits of adding pembrolizumab (Keytruda) for select patients battling advanced triple-negative breast cancer. Such robust R&D supporting PD-1 therapies is expected to drive market growth.
North American is Expected to Hold a Significant Market Share during the Forecast Period
North America is poised for substantial growth in the PD-1 and PD-L1 inhibitors market over the forecast period. This surge is attributed to the rising incidence of cancers, including skin cancer, urothelial carcinomas, and lung cancers, across the United States, Canada, Mexico, and other regional nations. Furthermore, the region's market expansion is bolstered by active product launches and ongoing research studies.
As the United States grapples with a rising number of melanoma cases, the demand for PD-1 and PD-L1 inhibitors is set to escalate, fueling regional market growth. For context, the American Society of Clinical Oncology's 2023 data projected that 97,610 adults in the United States would be diagnosed with invasive skin melanoma in 2023. Notably, melanoma ranks as the fifth most prevalent cancer in the United States, underscoring the swift uptick in its cases.
Intensified research on PD-1 inhibitors stands as a pivotal driver for market growth. For instance, at the IASLC 2023 World Conference on Lung Cancer in November 2023, results were presented from an analysis of two independent patient cohorts from the United States. These patients, diagnosed with advanced NSCLC and possessing a PD-L1 tumor proportion score (TPS) of 50% or higher, were treated with a PD-1 inhibitor. The findings underscored that PD-1 monotherapy offers a significant long-term survival advantage for patients with advanced NSCLC, especially those with a PD-L1 TPS of 90% or more.
Furthermore, a surge in product launches across the region is set to enhance drug availability, propelling market growth. For instance, in March 2023, the United States FDA greenlit Zynyz (retifanlimab-dlwr), a humanized monoclonal antibody targeting PD-1, for adults battling metastatic or recurrent locally advanced cell carcinoma. In a similar vein, the United States FDA, in September 2022, sanctioned the combination of durvalumab (Imfinzi, AstraZeneca UK Ltd) with gemcitabine and cisplatin for treating adults with locally advanced or metastatic biliary cancer (BTC).
Given these dynamics, the market is set for notable growth during the forecast period, driven by heightened R&D efforts and advancements in cancer treatments across the region.
PD-1 And PD-L1 Inhibitors Industry Overview
The PD-1 and PD-L1 inhibitors market is consolidated and consists of prominent market players. Some companies are expanding their market position by adopting various strategies such as acquisitions, mergers, and research collaboration, while others are investing in clinical trials to extend the treatment of other indications with the existing drugs to address the unmet challenges of the disease burden driving the market share. Some of the key market players in the PD-1 and PD-L1 inhibitors market are Bristol-Myers Squibb Company, Merck and Co., AstraZeneca PLC, Pfizer Inc., GSK plc, and F. Hoffmann-La Roche AG among others.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rising Investments in R&D and Clinical Trials by Bio-pharmaceutical Industries
- 4.2.2 Increased Encouragement Initiatives by the Regulatory Authorities with Favorable Approvals and Special Designations
- 4.2.3 Growing Burden of Different Cancers
- 4.3 Market Restraints
- 4.3.1 Risk of Complications Associated with the Highly Expensive Oncology Treatment
- 4.3.2 Challenges in Development with Uncertainty in Regulatory Process and High Costs of Tedious Clinical Trials
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Bargaining Power of Suppliers
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Threat of New Entrants
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD)
- 5.1 By Type of Inhibitors
- 5.1.1 PD-1 Inhibitors
- 5.1.2 PD-L1 Inhibitors
- 5.2 By Application
- 5.2.1 Hodgkins Lymphoma
- 5.2.2 Kidney Cancer
- 5.2.3 Melanoma
- 5.2.4 Non-small Cell Lung Cancer
- 5.2.5 Other Applications
- 5.3 By Distribution Channel
- 5.3.1 Hospital Pharmacies
- 5.3.2 Retail Pharmacies
- 5.3.3 Online Pharmacies
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United states
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Bristol-Myers Squibb Company
- 6.1.2 Merck & Co.
- 6.1.3 F. Hoffmann-La Roche AG
- 6.1.4 GSK plc
- 6.1.5 Amgen Inc.
- 6.1.6 Eli Lilly and Company
- 6.1.7 AstraZeneca PLC
- 6.1.8 BeiGene LTD
- 6.1.9 Pfizer Inc.
- 6.1.10 Regeneron Pharmaceuticals Inc.
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




