PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1406031

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1406031
GMP Testing Service - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts 2024 - 2029
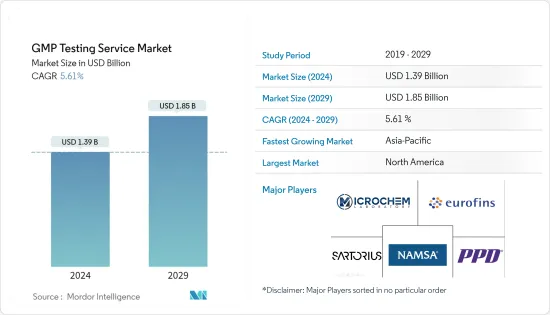
The GMP Testing Service Market size is estimated at USD 1.39 billion in 2024, and is expected to reach USD 1.85 billion by 2029, growing at a CAGR of 5.61% during the forecast period (2024-2029).
Lockdowns were implemented following the COVID-19 outbreak to stop the virus's spread. Both demand and supply-side effects on the industrial sector were seen as a result of these lockdowns. According to Eurostat 2021 from the European Commission, European industrial production climbed by 1.4% in July 2021, and the overall production level was 1% higher than in February 2020. The production of pharmaceutical goods and products increased by more than 30% between July 2020 and July 2021. However, businesses started to provide GMP-compliant coronavirus vaccine testing solutions for new viral threats and pandemics. It uses quick and sophisticated testing platforms, such as Next Generation Sequencing (NGS) technologies and more than 400 validated rapid PCR tests for potentially dangerous viruses in vaccine manufacturing processes. However, the market is growing at a stable pace due to a resumption of manufacturing activities. It is expected to witness a similar trend over the coming years.
Factors such as the growing pharmaceutical industry and increased drug and device development are expected to increase the market growth. For instance, according to the Indian Brand Equity Foundation Report 2022, India is the 12th largest exporter of medical goods globally. Indian drugs are exported to more than 200 countries worldwide, with the US being the key market. In addition, as per the same source mentioned above, generic drugs account for 20% of global exports in volume, making the country the largest provider of generic medicines globally. Indian drug and pharmaceutical exports stood at USD 24.60 billion in FY22 and USD 24.44 billion in FY21. Indian drug and pharmaceutical exports stood at USD 2,196.32 million in September 2022. Thus, growing pharmaceutical sectors are expected to boost demand for GMP testing services, boosting market growth.
The increasing manufacturing plant is expected to boost demand for GMP testing services, boosting market growth. For instance, in April 2022, Ferring Pharmaceuticals opened its integrated R&D and manufacturing facility in Hyderabad, which was established with a total investment of EUR 30 million (USD 32.73 million). The R&D capability includes formulation development, packaging development, and analytical development. The manufacturing facility is mainly designed for the solid oral dosage form.
While the above factors are expected to play a significant role in the GMP testing services market's growth, the regulatory framework's precise requirements concerning GMP outsourced activities may hinder the market's growth in the forecasted period.
GMP Testing Service Market Trends
Pharmaceutical and Biopharmaceutical Companies Segment Expected to Hold Significant Share in the GMP Testing Service Market
GMP is a system that guarantees goods are consistently produced and controlled by quality standards. The hazards associated with pharmaceutical production that cannot be removed through testing the finished product are intended to be reduced as much as possible. The pharmaceutical and biopharmaceutical companies segment is expected to grow during the forecast period.
The pharmaceutical and biopharmaceutical industries' innovative drug development is anticipated to fuel market expansion in this sector. According to the International Federation of Pharmaceutical Manufacturers and Associations' "Facts & Figures Report 2021," 24 were in Phase I, 34 were in Phase II, and 23 were in Phase III trials. The same report states that as of March 2021, the European Medicines Agency (EMA) had given Pfizer-BioNTech, Janssen, Moderna, and AstraZeneca's COVID-19 vaccines a conditional marketing authorization. The United States Food and Drug Administration (FDA) also approved COVID-19 vaccines from Pfizer BioNTech, Janssen, and Moderna. According to the Food and Drug Administration (FDA) Drug Recall Statistics published in July 2021, approximately 1,279 drugs are recalled each year globally, with 94% of FDA drug recalls occurring in the United States, followed by 4% in Canada. The FDA issued 12,028 drug recalls in the United States between 2012 and 2021. It necessitates the GMP testing of manufacturing plants and the assurance of public safety before they are marketed.
The strategic activities of the market players are expected to boost the market over the forecast period. For instance, in April 2021, PPD Inc., a leading global contract research organization, announced its plans to expand its Good Manufacturing Practice (GMP) lab in Ireland to enhance its biopharmaceutical testing capabilities. As a result, the category of pharmaceutical and biopharmaceutical businesses is driving market expansion. It is anticipated to favorably affect the GMP testing services market growth during the projected year.
North America is Expected to Hold Significant Share in the GMP testing Services Market Over the Forecast Period
North America is projected to hold a significant market share over the forecast period, owing to the presence of key market players and the availability of advanced technologies. Furthermore, favorable regulatory infrastructure and high medical demand are projected to boost regional market growth. FDA ensures product quality by closely monitoring drug manufacturers' compliance with its Current Good Manufacturing Practice (CGMP) regulations. The CGMP regulations for drugs include minimum requirements for the methods, facilities, and controls used in manufacturing, processing, and packaging a drug product. The regulations ensure a product is safe and contains the ingredients and strength it claims.
Moreover, the growing burden of chronic diseases is expected to spur increased demand for novel drugs, likely increasing drug manufacturing activities, thereby driving market growth. For instance, according to the 2022 data from the CDC, about six out of ten Americans suffer from chronic diseases, leading drivers of the nation's annual healthcare costs, amounting to around USD 4.1 trillion. The high disease burden is expected to increase demand for high-quality medicine, which leads to an increase in manufacturing activity that requires GMP testing, boosting the market growth.
The rising government investments in companies to improve their manufacturing technologies and the rising number of pharmaceutical manufacturers fueled market growth over the forecast period. For instance, in January 2021, Continuus Pharmaceuticals received a USD 69.3 million contract from the United States government to facilitate the domestic production of vital medicines to treat critically ill patients. Contract manufacturing organizations are expected to register a notable growth rate over the forecast period, owing to the increase in these organizations in developing regions. Moreover, the escalating number of drug approvals and the need for more manufacturing facilities to meet the studied market's demand are anticipated to boost the market's growth.
Thus, all the factors above are expected to propel the growth of the studied market over the forecast period.
GMP Testing Service Industry Overview
The GMP testing service market consists of several major players. A few major players dominate the market in terms of market share. The companies are taking initiatives to meet the higher demand for medicines and devices. The market players are adopting various strategies such as partnerships and collaboration to stay competitive in the market. Some of the companies currently dominating the market include Eurofins Scientific, PPD Inc., Microchem Laboratory, Sartorius AG, North American Science Associates Inc., Covance Inc., Nelson Laboratories LLC, Almac Group, Pace Analytical, Wuxi AppTec., Intertek Group PLC, and Charles River Laboratories.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Pharmaceutical Industry
- 4.2.2 Increasing Drug and Devices Development
- 4.3 Market Restraints
- 4.3.1 Stringent Regulatory Framework
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Bargaining Power of Suppliers
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Threat of New Entrants
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD)
- 5.1 By Service Type
- 5.1.1 Product Validation Testing
- 5.1.2 Bioanalytical Services
- 5.1.3 Packaging and Shelf-Life Testing
- 5.1.4 Other Service Types
- 5.2 By End User
- 5.2.1 Pharmaceutical and Biopharmaceutical Companies
- 5.2.2 Medical Devices Company
- 5.3 Geography
- 5.3.1 North America
- 5.3.1.1 United States
- 5.3.1.2 Canada
- 5.3.1.3 Mexico
- 5.3.2 Europe
- 5.3.2.1 Germany
- 5.3.2.2 United Kingdom
- 5.3.2.3 France
- 5.3.2.4 Italy
- 5.3.2.5 Spain
- 5.3.2.6 Rest of Europe
- 5.3.3 Asia-Pacific
- 5.3.3.1 China
- 5.3.3.2 Japan
- 5.3.3.3 India
- 5.3.3.4 Australia
- 5.3.3.5 South Korea
- 5.3.3.6 Rest of Asia-Pacific
- 5.3.4 Rest of the World
- 5.3.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Eurofins Scientific
- 6.1.2 PPD Inc.
- 6.1.3 Microchem Laboratory
- 6.1.4 Sartorius AG
- 6.1.5 North American Science Associates Inc.
- 6.1.6 Laboratory Corporation of America Holdings (Covance Inc.)
- 6.1.7 Sotera Health (Nelson Laboratories LLC)
- 6.1.8 Almac Group
- 6.1.9 Pace Analytical
- 6.1.10 Wuxi AppTec
- 6.1.11 Intertek Group PLC
- 6.1.12 Charles River Laboratories
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




