PUBLISHER: Roots Analysis | PRODUCT CODE: 1891246

PUBLISHER: Roots Analysis | PRODUCT CODE: 1891246
Vaccines Market: Industry Trends and Global Forecasts, Till 2035 - Distribution by Type of Vaccine API, Targeted Patient Population, Type of Vaccines, Route of Administration and Key Geographical Region
Vaccines Market: Overview
As per Roots Analysis, the vaccines market is estimated to grow from USD 48 billion in the current year to USD 94 billion by 2030, at a CAGR of 11.9% during the forecast period, till 2030.
Vaccines Market
The market sizing and opportunity analysis has been segmented across the following parameters:
Type of Vaccine API
- Conjugate Vaccines
- Inactivated and Subunit Vaccines
- Live Attenuated Vaccines
- Recombinant Vaccines
- Toxoid Vaccines
- Others
Targeted Patient Population
- Pediatric
- Adult
Type of Vaccines
- Pneumococcal Conjugate Vaccine
- Human Papillomavirus Vaccine
- Influenza Vaccine
- Rotavirus Vaccine
- Varicella Vaccine
- DTP-HepB-Hib Vaccine
- Others
Route of Administration
- Intramuscular
- Subcutaneous
- Oral Administration
- Intravenous
- Others
Key Geographical Region
- North America
- Europe
- Rest of the World
Vaccines Market: Growth and Trends
With the increasing threat of infectious diseases, there has been a rise in the need for vaccines worldwide. A vaccine is a type of biological formulation created using weakened or inactive microbes, along with their surface proteins and toxins. These vaccines offer active acquired immunity against specific diseases and allow the immune system to produce a robust response if a person encounters the same pathogen in the future. Vaccination is now a critical means of lowering the risk of infectious diseases and decreasing global mortality rates. The global emergence of infectious diseases has highlighted the need for improved vaccines to mitigate disease risks. Recently, the World Health Organization published a report on disease outbreaks, revealing the rising number of people affected by Avian influenza A and Mpox.
Moreover, the European Centre for Disease Prevention and Control reported that approximately 4.5 million dengue cases had been documented worldwide, with about 4,000 fatalities confirmed. In addition, the World Health Organization announced approximately 64 new infectious diseases impacting individuals worldwide. Further, approximately 2 million individuals affected by rotavirus are admitted to the hospital annually. In this context, numerous industry leaders, governmental bodies, and public health organizations have launched diverse immunization initiatives and concentrated on creating advanced vaccines. In was also recently reported that Biovac and Sanfoi established a collaboration for the manufacturing of inactivated polio vaccines in Africa. The aim of the collaboration is to increase the production of polio vaccines to satisfy the rising need for these vaccines in more than 40 African nations.
Additionally, French President Emmanuel Macron opted to collaborate with several African leaders in organizing a USD 1.1 billion initiative aimed at speeding up vaccine production in African nations. At present, various research projects have been launched by stakeholders to promote innovation in the healthcare sector and reduce the risk of transmitting infectious diseases. Due to the continuous need for vaccines and rising collaborations, the vaccine market is expected to expand at a consistent pace during the forecast period.
Vaccines Market: Key Insights
The report delves into the current state of the vaccines market and identifies potential growth opportunities within industry. Some key findings from the report include:
- More than 200 preventive vaccines, developed by both industry and non-industry players, are being evaluated in clinical stages of development.
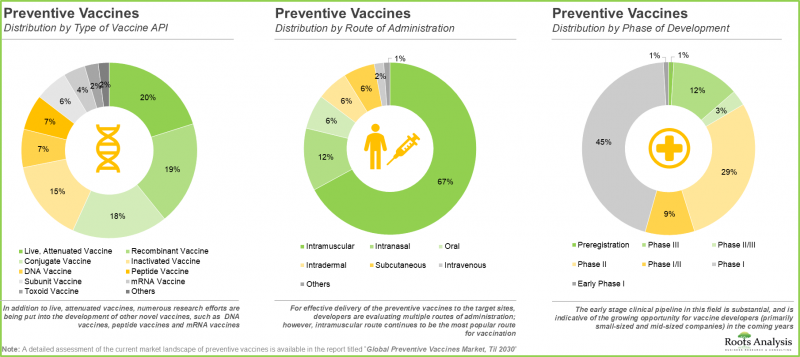
- A variety of vaccine APIs, designed for administration via multiple routes of delivery, are presently being investigated; most such candidates are in the early stages of development.
- In order to achieve a competitive edge, vaccine developers are putting in significant efforts to ensure that their candidates are clinically and commercially competent.
- Foreseeing a lucrative future in this domain, several private and public investors have invested over USD 10 billion in vaccine development initiatives, across 175 instances, in the time period since 2015.
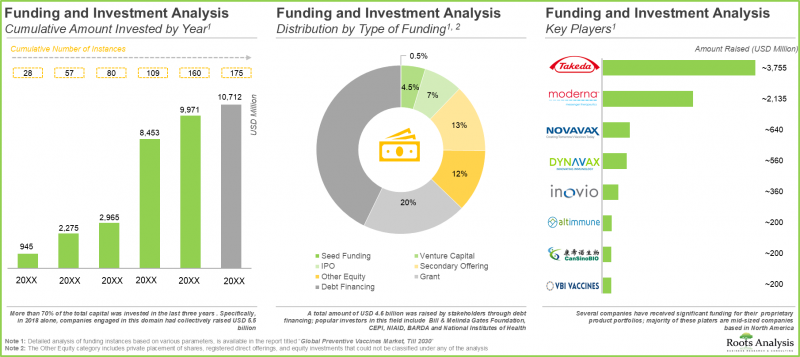
- Over the last few years, 1,400+ clinical trials evaluating various types of preventive vaccines have been registered, indicating the rapid pace of development in this field.
- Around 70 companies, situated in different regions across the globe, claim to provide contract development, fill / finish and regulatory support, in addition to manufacturing services.
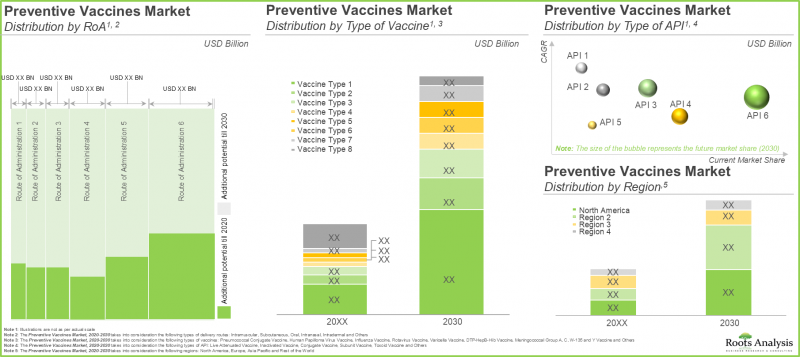
- The market is anticipated to grow at a CAGR of 11.9%, till 2030, and the projected opportunity is likely to be distributed across various routes of administration, vaccine types and key geographical regions.
Vaccines Market: Key Segments
Live Attenuated Vaccines Segment is Likely to Capture the Majority of the Market Share
In terms of the type of technology, the global market for vaccines is segmented into conjugate vaccines, inactivated and subunit vaccines, live attenuated vaccines, recombinant vaccines, toxoid vaccines, and others. Fueled by the rising need for potent vaccines to combat various infectious diseases, live attenuated vaccines are projected to occupy the largest segment, securing 28% of the total vaccines market share in the current year. Other types of vaccines will grow at a higher CAGR of 16.8% during the forecast period owing to the growing research on novel vaccines such as DNA vaccines.
Pediatric Segment Holds the Highest Vaccines Market Share
In terms of target patient population, the global market has been distributed into pediatrics and adults. According to our market study, the pediatric segment accounts for the largest share (56%) of the vaccines market in the current year. The expansion of the pediatric market segment is due to the rising emphasis on immunizing infants against illnesses and the increasing government efforts to promote vaccination among children. In the long run, the adult segment will grow at a higher CAGR of 12.1% during the forecast period, driven by the increasing cases of endemic and pandemics.
The Current Market Share is Captured by Intramuscular Segment
In terms of route of administration, the vaccines market is segmented into intramuscular, subcutaneous, oral, intravenous and others. According to our projection, the intramuscular segment capture majority (52%) of the overall vaccine market in the current year. The increase can be attributed to the rising preference for parenteral vaccines, as they can avoid the gastrointestinal tract. The intramuscular route facilitates easy administration and produces fewer side effects. In the long term, the intravenous segment is expected to grow at a higher CAGR of 14.6% throughout the forecast period.
MMR Vaccines will Grow at a Higher CAGR of 12.3% during the Forecast Period
In terms of the type of vaccines, the market is segmented into Pneumococcal Conjugate Vaccine, Human Papillomavirus Vaccine, Rotavirus Vaccine, Influenza Vaccine, MMR Vaccine, Tetanus and Diphtheria Booster Vaccine, Varicella Vaccine, DTaP-Hib-IPV Vaccine, DTaP-HepB-Hib-IPV Vaccine and other. Driven by the growing incidences of infectious diseases, the others segment accounts to hold the largest (32.4%) vaccines market share in the current year. Additionally, the pneumococcal conjugate vaccine accounts to hold the second highest share 25.8% of the market currently. Pfizer with its Prevnar series and Merck are the market leaders in pneumococcal vaccine market. Merck received US FDA approval for its 21-valent pneumococcal vaccine, CAPVAXIVE recently. In the long run, MMR vaccines will grow at a higher CAGR of 12.3% during the forecast period.
North America Accounts for the Highest Revenues in the Global Vaccines Market in the Current Year
In terms of key geographies, the global market has been distributed across North America, Europe, Asia-Pacific and Rest of the World. According to our projections, North America accounts for the largest share (44%) of the market in the current year. The primary factors driving the growth of the vaccine market in this area are the heightened focus on vaccination implementation, the fast-expanding healthcare industry, and a rise in vaccine research in the US. However, the demand for vaccines in Asia-Pacific is estimated to witness growth and is likely to grow at a substantial CAGR of 14.7% during the forecast period. In February 2023, the Government of India forged a new three-year partnership with Gavi (Leading Vaccine Alliance) with the aim of immunizing millions of children in India with appropriate vaccines. Under this agreement, Gavi has allocated USD 250 million for the identification and vaccination of children who haven't received any vaccine strengthening healthcare systems and supporting the introduction of HPV (human papillomavirus vaccine) along with TCV (typhoid conjugate vaccine) into India's national routine immunization schedule.
Market Share by Key Players
The key players active in this industry are Bio Farma, Emergent BioSolutions, GC Pharma, GlaxoSmithKline, Janssen, Merck, Novavax, Moderna, Pfizer, Sanofi Pasteur and Valneva. Currently, GlaxoSmithKline accounts to hold 21% of the overall vaccines market share in the current year. The highest share can be attributed to its extensive range of vaccines and GlaxoSmithKline's acceleration of research and development efforts in the vaccine sector.
Example Players in Vaccines Market
- Bio Farma
- Emergent BioSolutions
- GC Pharma
- GlaxoSmithKline
- Janssen
- Merck
- Novavax
- Moderna
- Pfizer
- Sanofi Pasteur
- Valneva
Vaccines Market: Research Coverage
- Market Sizing and Opportunity Analysis: The report features an in-depth analysis of the vaccines market, focusing on key market segments, including [A] type of vaccine API, [B] targeted patient population, [C] type of vaccines, [D] route of administration, and [E] key geographical regions.
- Market Landscape: A detailed assessment of the current market landscape of over 200 preventive vaccines that are currently being evaluated in different stages of development, based on a number of parameters, such as [A] type of developer, [B] phase of development of lead candidates, [C] route of administration, [D] type of vaccine API, [E] dosage form, dosage, [F] target disease indication and [G] target patient population.
- Company Competitiveness Analysis: A comprehensive competitive analysis of of preventive vaccine developers based in North America, Europe and Asia-Pacific, examining factors, such as [A] supplier strength and [B] pipeline strength.
- Company Profiles: In-depth profiles of the key preventive vaccine developers based in North America, Europe and Asia-Pacific that are engaged in manufacturing focusing on [A] year of establishment, [B] location of headquarters, [C] product portfolio, [D] recent developments and [E] an informed future outlook.
- Clinical Trial Analysis: A detailed analysis of various completed, ongoing and planned clinical studies of preventive vaccines based on various relevant parameters, including [A] trial registration year, [B] phase of development, [C] trial recruitment status, [D] study design, [E] trial focus area, [F] type of preventive vaccine, [G] target disease indication(s), [H] type of sponsor / collaborator, [I] leading industry sponsors / collaborators, [J] enrolled patients population and [K] regional distribution.
- Ongoing Vaccine Development Initiatives for Complex Conditions: An overview of the ongoing vaccine development initiatives for complex conditions, such as [A] COVID-19, [B] Ebola virus disease, [C] HIV / AIDS, [D] malaria and [E] zika virus infection, including information on disease, its global burden, current treatment landscape and preventive vaccine research landscape.
Key Questions Answered in this Report
- How many companies are currently engaged in this market?
- Which are the leading companies in this market?
- What is the current global capacity of developers?
- What factors are likely to influence the evolution of this market?
- What is the current and future market size?
- What is the CAGR of this market?
- How is the current and future market opportunity likely to be distributed across key market segments?
Reasons to Buy this Report
- The report provides a comprehensive market analysis, offering detailed revenue projections of the overall market and its specific sub-segments. This information is valuable to both established market leaders and emerging entrants.
- Stakeholders can leverage the report to gain a deeper understanding of the competitive dynamics within the market. By analyzing the competitive landscape, businesses can make informed decisions to optimize their market positioning and develop effective go-to-market strategies.
- The report offers stakeholders a comprehensive overview of the market, including key drivers, barriers, opportunities, and challenges. This information empowers stakeholders to stay abreast of market trends and make data-driven decisions to capitalize on growth prospects.
Additional Benefits
- Complimentary PPT Insights Packs
- Complimentary Excel Data Packs for all Analytical Modules in the Report
- 15% Free Content Customization
- Detailed Report Walkthrough Session with Research Team
- Free Updated report if the report is 6-12 months old or older
TABLE OF CONTENTS
1. PREFACE
- 1.1. Scope of the Report
- 1.2. Research Methodology
- 1.3. Chapter Outlines
2. EXECUTIVE SUMMARY
3. INTRODUCTION
- 3.1. Chapter Overview
- 3.2. Preventive Vaccines
- 3.2.1. Classification of Vaccines
- 3.2.1.1. Live, Attenuated Vaccines
- 3.2.1.2. Inactivated Vaccines
- 3.2.1.3. Subunit Vaccines
- 3.2.1.4. Toxoid Vaccines
- 3.2.1.5. DNA Vaccines
- 3.2.2. Key Components of a Vaccine Formulation
- 3.2.3. Production of Vaccines using Different Expression Systems
- 3.2.3.1. Embryonated Chicken Eggs and Primary Chicken Embryonic Fibroblasts (CEFs)
- 3.2.3.2. Mammalian Expression Systems
- 3.2.3.3. Avian Expression Systems
- 3.2.3.4. Plant Expression Systems
- 3.2.3.5. Bacterial Expression Systems
- 3.2.3.6. Yeast Expression Systems
- 3.2.3.7. Insect Expression System
- 3.2.4. Routes of Vaccine Administration
- 3.2.4.1. Intramuscular Route
- 3.2.4.2. Subcutaneous Route
- 3.2.4.3. Oral Route
- 3.2.4.4. Intranasal Route
- 3.2.4.5. Intradermal Route
- 3.2.4.6. Inhalation
- 3.2.5. Clinical Development and Approval of Vaccines
- 3.2.6. Future Perspectives
- 3.2.1. Classification of Vaccines
4. MARKET LANDSCAPE
- 4.1. Chapter Overview
- 4.2. Preventive Vaccines: Overall Market Landscape
- 4.2.1. Marketed Vaccines Landscape
- 4.2.2. Clinical-Stage Vaccines Landscape
- 4.2.2.1. Analysis by Type of Developer
- 4.2.2.2. Analysis by Phase of Development
- 4.2.2.3. Analysis by Route of Administration
- 4.2.2.4. Analysis by Type of Vaccine API
- 4.2.2.5. Analysis by Dosage Form
- 4.2.2.6. Analysis by Dosage
- 4.2.2.7. Analysis by Target Disease Indication
- 4.2.2.8. Analysis by Target Patient Population
- 4.2.2.9. Key Industry Players: Analysis by Number of Vaccines in Clinical Development
- 4.2.2.10. Key Non-Industry Players: Analysis by Number of Vaccines in Clinical Development
5. COMPANY COMPETITIVENESS ANALYSIS
- 5.1. Chapter Overview
- 5.2. Methodology
- 5.3. Assumptions and Key Parameters
- 5.4. Competitiveness Analysis: Preventive Vaccine Developers
- 5.4.1. Preventive Vaccine Developers based in North America
- 5.4.2. Preventive Vaccine Developers based in Europe
- 5.4.3. Preventive Vaccine Developers based in Asia Pacific
6. COMPANY PROFILES
- 6.1. Chapter Overview
- 6.2. Bio Farma
- 6.2.1. Company Overview
- 6.2.2. Preventive Vaccines Portfolio
- 6.2.3. Recent Developments and Future Outlook
- 6.3. Emergent BioSolutions
- 6.3.1. Company Overview
- 6.3.2. Preventive Vaccines Portfolio
- 6.3.3. Recent Developments and Future Outlook
- 6.4. GC Pharma
- 6.4.1. Company Overview
- 6.4.2. Preventive Vaccines Portfolio
- 6.4.3. Recent Developments and Future Outlook
- 6.5. GlaxoSmithKline
- 6.5.1. Company Overview
- 6.5.2. Preventive Vaccines Portfolio
- 6.5.3. Recent Developments and Future Outlook
- 6.6. Janssen
- 6.6.1. Company Overview
- 6.6.2. Preventive Vaccines Portfolio
- 6.6.3. Recent Developments and Future Outlook
- 6.7. Merck
- 6.7.1. Company Overview
- 6.7.2. Preventive Vaccines Portfolio
- 6.7.3. Recent Developments and Future Outlook
- 6.8. Novavax
- 6.8.1. Company Overview
- 6.8.2. Preventive Vaccines Portfolio
- 6.8.3. Recent Developments and Future Outlook
- 6.9. Pfizer
- 6.9.1. Company Overview
- 6.9.2. Preventive Vaccines Portfolio
- 6.9.3. Recent Developments and Future Outlook
- 6.10. Sanofi Pasteur
- 6.10.1. Company Overview
- 6.10.2. Preventive Vaccines Portfolio
- 6.10.3. Recent Developments and Future Outlook
- 6.11. Valneva
- 6.11.1. Company Overview
- 6.11.2. Preventive Vaccines Portfolio
- 6.11.3. Recent Developments and Future Outlook
7. CLINICAL TRIAL ANALYSIS
- 7.1. Chapter Overview
- 7.2. Scope and Methodology
- 7.3. Preventive Vaccines: Clinical Trial Analysis
- 7.3.1. Analysis by Trial Registration Year
- 7.3.2. Analysis by Enrolled Patient Population and Trial Registration Year
- 7.3.3. Analysis by Trial Phase
- 7.3.4. Analysis by Trial Recruitment Status
- 7.3.5. Analysis by Study Design
- 7.3.6. Analysis by Trial Focus Area
- 7.3.7. Analysis by Type of Preventive Vaccine (based on Pathogen)
- 7.3.8. Analysis by Target Disease Indication
- 7.3.9. Analysis by Type of Sponsor / Collaborator
- 7.3.10. Leading Industry Players: Analysis by Number of Registered Trials
- 7.3.11. Geographical Analysis by Number of Registered Trials
- 7.3.12. Geographical Analysis by Enrolled Patient Population
- 7.3.13. Geographical Analysis by Trial Recruitment Status
8. ONGOING VACCINE DEVELOPMENT INITIATIVES FOR COMPLEX CONDITIONS
- 8.1. Chapter Overview
- 8.2. Coronavirus Disease (COVID-19)
- 8.2.1. Disease Overview
- 8.2.2. Global Burden of COVID-19
- 8.2.3. Current Treatment Landscape
- 8.2.4. Preventive Vaccines for COVID-19
- 8.2.4.1. Historical Background of COVID-19 Vaccine Research
- 8.2.4.2. COVID-19 and Affiliated Research Landscape
- 8.2.5. Funding Instances
- 8.2.6. Recent Developments
- 8.3. Ebola Virus Disease (EVD)
- 8.3.1. Disease Overview
- 8.3.2. Global Burden of EVD
- 8.3.3. Current Treatment Landscape
- 8.3.4. Preventive Vaccines for EVD
- 8.3.4.1. Historical Background of Ebola Virus Vaccine Research
- 8.3.4.2. Anti-Ebola Virus Vaccines and Affiliated Research Landscape
- 8.3.5. Funding Instances
- 8.3.6. Recent Developments
- 8.4. HIV/AIDS
- 8.4.1. Disease Overview
- 8.4.2. Global Burden of HIV/AIDS
- 8.4.3. Current Treatment Landscape
- 8.4.4. Preventive Vaccines for HIV/AIDS
- 8.4.4.1. Historical Background of HIV/AIDS Vaccine Research
- 8.4.4.2. Anti-HIV Vaccines and Affiliated Research Landscape
- 8.4.5. Funding Instances
- 8.4.6. Recent Developments
- 8.5. Malaria
- 8.5.1. Disease Overview
- 8.5.2. Global Burden of Malaria
- 8.5.3. Current Treatment Landscape
- 8.5.4. Preventive Vaccines for Malaria
- 8.5.4.1. Historical Background of Malaria Vaccine Research
- 8.5.4.2. Anti-Malaria Vaccines and Affiliated Research Landscape
- 8.5.5. Funding Instances
- 8.5.6. Recent Developments
- 8.6. Zika Virus Infection
- 8.6.1. Disease Overview
- 8.6.2. Global Burden of Zika Virus Infection
- 8.6.3. Current Treatment Landscape
- 8.6.4. Preventive Vaccines for Zika Virus Infection
- 8.6.4.1. Historical Background of Zika Virus Vaccine Research
- 8.6.4.2. Anti-Zika Virus Vaccines and Affiliated Research Landscape
- 8.6.5. Funding Instances
- 8.6.6. Recent Developments
9. FUNDING AND INVESTMENT ANALYSIS
- 9.1. Chapter Overview
- 9.2. Types of Funding
- 9.3. Preventive Vaccines: Funding and Investment Analysis
- 9.3.1. Analysis by Number of Funding Instances
- 9.3.2. Analysis by Amount Invested
- 9.3.3. Analysis by Type of Funding
- 9.3.4. Analysis by Amount Invested across Different Types of Vaccine API
- 9.3.5. Analysis by Focus Area
- 9.3.6. Analysis by Amount Invested by Different Type of Investors
- 9.3.7. Most Active Players: Analysis by Number of Funding Instances
- 9.3.8. Most Active Investors: Analysis by Number of Funding Instances
- 9.3.9. Analysis by Geography
- 9.3.9.1. Continent-wise Analysis
- 9.3.9.2. Country-wise Analysis
10. MARKET SIZING AND OPPORTUNITY ANALYSIS
- 10.1. Chapter Overview
- 10.2. Forecast Methodology and Key Assumptions
- 10.3. Overall Preventive Vaccines Market, till 2030
- 10.3.1. Preventive Vaccines Market, till 2030: Distribution by Route of Administration
- 10.3.2. Preventive Vaccines Market, till 2030: Distribution by Type of Vaccine
- 10.3.3. Preventive Vaccines Market, till 2030: Distribution by Type of Vaccine API
- 10.3.4. Preventive Vaccines Market, till 2030: Distribution by Target Patient Population
- 10.3.5. Preventive Vaccines Market, till 2030: Distribution by Key Geographical Regions
- 10.3.5.1. Preventive Vaccines Market in North America, till 2030
- 10.3.5.1.1. Preventive Vaccines Market in the US, till 2030
- 10.3.5.1.2. Preventive Vaccines Market in Mexico, till 2030
- 10.3.5.1.3. Preventive Vaccines Market in Canada, till 2030
- 10.3.5.2. Preventive Vaccines Market in Europe, till 2030
- 10.3.5.2.1. Preventive Vaccines Market in Spain, till 2030
- 10.3.5.2.2. Preventive Vaccines Market in the UK, till 2030
- 10.3.5.2.3. Preventive Vaccines Market in Italy, till 2030
- 10.3.5.2.4. Preventive Vaccines Market in France, till 2030
- 10.3.5.2.5. Preventive Vaccines Market in Germany, till 2030
- 10.3.5.2.6. Preventive Vaccines Market in Rest of Europe, till 2030
- 10.3.5.3. Preventive Vaccines Market in Asia Pacific, till 2030
- 10.3.5.3.1. Preventive Vaccines Market in India, till 2030
- 10.3.5.3.2. Preventive Vaccines Market in China, till 2030
- 10.3.5.3.3. Preventive Vaccines Market in Australia, till 2030
- 10.3.5.3.4. Preventive Vaccines Market in Rest of Asia Pacific, till 2030
- 10.3.5.4. Preventive Vaccines Market in Rest of the World, till 2030
- 10.3.5.1. Preventive Vaccines Market in North America, till 2030
11. CASE-IN-POINT: CONTRACT MANUFACTURING OF VACCINES
- 11.1. Chapter Overview
- 11.2. Vaccine Contract Manufacturing
- 11.2.1. Addressing an Unmet Need
- 11.2.2. Commonly Outsourced Operations
- 11.2.3. Selecting a CMO Partner
- 11.2.4. Advantages of Outsourcing Manufacturing Services
- 11.2.5. Associated Risks and Challenges
- 11.3. Vaccine Contract Manufacturing: Overall Market Landscape
- 11.3.1. Analysis by Year of Establishment
- 11.3.2. Analysis by Company Size
- 11.3.3. Analysis by Scale of Operation
- 11.3.4. Analysis by Location of Headquarters
- 11.3.5. Analysis by Location of Manufacturing Facilities
- 11.3.6. Analysis by Type of Service(s) Offered
- 11.3.7. Analysis by Expression System Used
- 11.3.8. Analysis by Type of Vaccine Manufactured
- 11.3.9. Analysis by Type of Vaccine Manufactured and Location of Headquarters
12. CONCLUDING REMARKS
13. EXECUTIVE INSIGHTS
- 13.1. Chapter Overview
- 13.2. Company A
- 13.2.1. Company Snapshot
- 13.2.2. Interview Transcript: Chief Executive Officer
14. APPENDIX 1: TABULATED DATA
15. APPENDIX 2: LIST OF COMPANIES AND ORGANIZATIONS
List of Tables
- Table 3.1 Classification of Vaccines
- Table 3.2 Live, Attenuated Vaccines: Commonly Reported Adverse Events
- Table 3.3 Inactivated Vaccines: Commonly Reported Adverse Events
- Table 3.4 Subunit Vaccines: Commonly Reported Adverse Events
- Table 3.5 Toxoid Vaccines: Commonly Reported Adverse Events
- Table 3.6 Vaccine Excipients and their Functions
- Table 3.7 Routes of Administration and Type of Delivery Devices for Vaccine Administration
- Table 3.8 Common Pediatric Vaccines and their Routes of Administration
- Table 4.1 List of Marketed Preventive Vaccines
- Table 4.2 List of Clinical-Stage Preventive Vaccines
- Table 6.1 Preventive Vaccine Developers: List of Companies Profiled
- Table 6.2 Bio Farma: Company Overview
- Table 6.3 Bio Farma: Preventive Vaccine Pipeline
- Table 6.4 Bio Farma: Recent Developments and Future Outlook
- Table 6.5 Emergent BioSolutions: Company Overview
- Table 6.6 Emergent BioSolutions: Preventive Vaccine Pipeline
- Table 6.7 Emergent BioSolutions: Recent Developments and Future Outlook
- Table 6.8 GC Pharma: Company Overview
- Table 6.9 GC Pharma: Preventive Vaccine Pipeline
- Table 6.10 GC Pharma: Recent Developments and Future Outlook
- Table 6.11 GlaxoSmithKline: Company Overview
- Table 6.12 GlaxoSmithKline: Preventive Vaccine Pipeline
- Table 6.13 GlaxoSmithKline: Recent Developments and Future Outlook
- Table 6.14 Janssen: Company Overview
- Table 6.15 Janssen: Preventive Vaccine Pipeline
- Table 6.16 Janssen: Recent Developments and Future Outlook
- Table 6.17 Merck: Company Overview
- Table 6.18 Merck: Preventive Vaccine Pipeline
- Table 6.19 Merck: Recent Developments and Future Outlook
- Table 6.20 Novavax: Company Overview
- Table 6.21 Novavax: Preventive Vaccine Pipeline
- Table 6.22 Novavax: Recent Developments and Future Outlook
- Table 6.23 Pfizer: Company Overview
- Table 6.24 Pfizer: Preventive Vaccine Pipeline
- Table 6.25 Pfizer: Recent Developments and Future Outlook
- Table 6.26 Sanofi Pasteur: Company Overview
- Table 6.27 Sanofi Pasteur: Preventive Vaccine Pipeline
- Table 6.28 Sanofi Pasteur: Recent Developments and Future Outlook
- Table 6.29 Valneva: Company Overview
- Table 6.30 Valneva: Preventive Vaccine Pipeline
- Table 6.31 Valneva: Recent Developments and Future Outlook
- Table 8.1 Preventive Vaccines under Investigation for COVID-19
- Table 8.2 COVID-19 Vaccines: Funding Instances
- Table 8.3 Ebola Virus Disease: List of Marketed Therapeutics
- Table 8.4 Preventive Vaccines under Investigation for Ebola Virus Disease
- Table 8.5 Anti-Ebola Virus Vaccines: Funding Instances
- Table 8.6 HIV/AIDS: List of Marketed Therapeutics
- Table 8.7 Preventive Vaccines under Investigation for HIV/AIDS
- Table 8.8 Anti-HIV Vaccines: Funding Instances
- Table 8.9 Malaria: List of Marketed Therapeutics
- Table 8.10 Preventive Vaccines under Investigation for Malaria
- Table 8.11 Anti-Malaria Vaccines: Funding Instances
- Table 8.12 Preventive Vaccines under Investigation for Zika Virus Infection
- Table 8.13 Anti-Zika Virus Vaccines: Funding Instances
- Table 9.1 Preventive Vaccines: List of Funding and Investments
- Table 11.1 Vaccine Contract Manufacturers: List of Service Providers
- Table 11.2 Vaccine CMOs: Information on Type of Services Offered
- Table 11.3 Vaccine CMOs: Information on Scale of Operation
- Table 11.4 Vaccine CMOs: Information on Expression System Used
- Table 11.5 Vaccine CMOs: Information on Type of Vaccines Manufactured
- Table 11.6 List of Ad hoc Vaccine Manufacturers
- Table 12.1 Preventive Vaccines Market: Summary of the Report
- Table 14.1 Clinical-Stage Preventive Vaccines: Distribution by Type of Developer
- Table 14.2 Clinical-Stage Preventive Vaccines: Distribution by Phase of Development
- Table 14.3 Clinical-Stage Preventive Vaccines: Distribution by Route of Administration
- Table 14.4 Clinical-Stage Preventive Vaccines: Distribution by Type of Vaccine API
- Table 14.5 Clinical-Stage Preventive Vaccines: Distribution by Type of Vaccine API and Phase of Development
- Table 14.6 Clinical-Stage Preventive Vaccines: Distribution by Dosage Form
- Table 14.7 Clinical-Stage Preventive Vaccines: Distribution by Dosage
- Table 14.8 Clinical-Stage Preventive Vaccines: Distribution by Target Disease Indication
- Table 14.9 Clinical-Stage Preventive Vaccines: Distribution by Target Patient Population
- Table 14.10 Key Industry Players: Distribution by Number of Vaccines in Clinical Development
- Table 14.11 Key Non-Industry Players: Distribution by Number of Vaccines in Clinical Development
- Table 14.12 Clinical Trial Analysis: Cumulative Distribution by Trial Registration Year, Since 2010
- Table 14.13 Clinical Trial Analysis: Distribution by Number of Patients Enrolled by Trial Registration Year, Since 2010
- Table 14.14 Clinical Trial Analysis: Distribution by Trial Phase
- Table 14.15 Clinical Trial Analysis: Distribution by Trial Recruitment Status
- Table 14.16 Clinical Trial Analysis: Cumulative Year-wise Trend by Trial Recruitment Status
- Table 14.17 Clinical Trial Analysis: Distribution by Study Design
- Table 14.18 Clinical Trial Analysis: Distribution by Trial Focus Area
- Table 14.19 Clinical Trial Analysis: Distribution by Type of Preventive Vaccine (based on Pathogen)
- Table 14.20 Clinical Trial Analysis: Cumulative Year-wise Trend by Type of Preventive Vaccines
- Table 14.21 Clinical Trial Analysis: Distribution by Target Disease Indication
- Table 14.22 Clinical Trial Analysis: Distribution by Patient Enrollment and Target Disease Indication
- Table 14.23 Clinical Trial Analysis: Distribution by Type of Sponsor / Collaborator
- Table 14.24 Leading Industry Players: Distribution by Number of Registered Trials
- Table 14.25 Funding and Investment Analysis: Cumulative Number of Instances by Year, Since 2015
- Table 14.26 Funding and Investment Analysis: Cumulative Amount Invested, Since 2015 (USD Million)
- Table 14.27 Funding and Investment Analysis: Distribution by Type of Funding, Since 2015
- Table 14.28 Funding and Investment Analysis: Distribution of Amount Invested by Type of Funding, Since 2015 (USD Million)
- Table 14.29 Funding and Investment Analysis: Distribution of Amount Invested by Type of Vaccine API
- Table 14.30 Funding and Investment Analysis: Distribution of Amount Invested by Focus Area
- Table 14.31 Funding and Investment Analysis: Distribution of Amount Invested by Different Type of Investors
- Table 14.32 Most Active Players: Distribution by Number of Funding Instances
- Table 14.33 Most Active Investors: Distribution by Number of Funding Instances
- Table 14.34 Funding and Investment Analysis: Regional Distribution by Number of Funding Instances and Amount Raised (USD Million)
- Table 14.35 Funding and Investment Analysis: Country-wise Distribution by Total Amount Invested (USD Million)
- Table 14.36 Overall Preventive Vaccines Market, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.37 Preventive Vaccines Market, Till 2030: Distribution by Route of Administration (USD Billion)
- Table 14.38 Preventive Vaccines Market, Till 2030: Distribution by Type of Vaccine (USD Billion)
- Table 14.39 Preventive Vaccines Market, Till 2030: Distribution by Type of Vaccine API (USD Billion)
- Table 14.40 Preventive Vaccines Market, Till 2030: Distribution by Target Patient Population (USD Billion)
- Table 14.41 Preventive Vaccines Market, Till 2030: Distribution by Key Geographical Regions (USD Billion)
- Table 14.42 Preventive Vaccines Market in North America, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.43 Preventive Vaccines Market in the US, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.44 Preventive Vaccines Market in Mexico, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.45 Preventive Vaccines Market in Canada, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.46 Preventive Vaccines Market in Europe, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.47 Preventive Vaccines Market in Spain, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.48 Preventive Vaccines Market in the UK, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.49 Preventive Vaccines Market in Italy, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.50 Preventive Vaccines Market in France, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.51 Preventive Vaccines Market in Germany, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.52 Preventive Vaccines Market in Rest of Europe, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.53 Preventive Vaccines Market in Asia Pacific, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.54 Preventive Vaccines Market in India, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.55 Preventive Vaccines Market in China, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.56 Preventive Vaccines Market in Australia, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.57 Preventive Vaccines Market in Rest of Asia Pacific, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.58 Preventive Vaccines Market in Rest of the World, Conservative, Base and Optimistic Scenarios, Till 2030 (USD Billion)
- Table 14.59 Vaccine CMOs: Distribution by Year of Establishment
- Table 14.60 Vaccine CMOs: Distribution by Company Size
- Table 14.61 Vaccine CMOs: Distribution by Scale of Operation
- Table 14.62 Vaccine CMOs: Distribution by Location of Headquarters (Region and Country-wise)
- Table 14.63 Vaccine CMOs: Distribution by Location of Manufacturing Facility (Region-wise)
- Table 14.64 Vaccine CMOs: Distribution by Type of Services Offered
- Table 14.65 Vaccine CMOs: Distribution by Expression System Used
- Table 14.66 Vaccine CMOs: Distribution by Type of Vaccines Manufactured
- Table 14.67 Vaccine CMOs: Region-wise Distribution by Type of Vaccine Manufactured
List of Figures
- Figure 3.1 Difference Between Vaccines and Small Molecules
- Figure 3.2 Classification of Vaccines
- Figure 3.3 Routes for Vaccine Administration
- Figure 4.1 Clinical-Stage Preventive Vaccines: Distribution by Type of Developer
- Figure 4.2 Clinical-Stage Preventive Vaccines: Distribution by Phase of Development
- Figure 4.3 Clinical-Stage Preventive Vaccines: Distribution by Route of Administration
- Figure 4.4 Clinical-Stage Preventive Vaccines: Distribution by Type of Vaccine API
- Figure 4.5 Clinical-Stage Preventive Vaccines: Distribution by Type of Vaccine API and Phase of Development
- Figure 4.6 Clinical-Stage Preventive Vaccines: Distribution by Dosage Form
- Figure 4.7 Clinical-Stage Preventive Vaccines: Distribution by Dosage
- Figure 4.8 Clinical-Stage Preventive Vaccines: Distribution by Target Disease Indication
- Figure 4.9 Clinical-Stage Preventive Vaccines: Distribution by Target Patient Population
- Figure 4.10 Key Industry Players: Distribution by Number of Vaccines in Clinical Development
- Figure 4.11 Key Non-Industry Players: Distribution by Number of Vaccines in Clinical Development
- Figure 5.1 Company Competitiveness Analysis: Preventive Vaccine Developers based in North America
- Figure 5.2 Company Competitiveness Analysis: Preventive Vaccine Developers based in Europe
- Figure 5.3 Company Competitiveness Analysis: Preventive Vaccine Developers based in Asia Pacific
- Figure 7.1 Clinical Trial Analysis: Scope and Methodology
- Figure 7.2 Clinical Trial Analysis: Cumulative Distribution by Trial Registration Year, Since 2010
- Figure 7.3 Clinical Trial Analysis: Distribution by Number of Patients Enrolled by Trial Registration Year, Since 2010
- Figure 7.4 Clinical Trial Analysis: Distribution by Trial Phase
- Figure 7.5 Clinical Trial Analysis: Distribution by Trial Recruitment Status
- Figure 7.6 Clinical Trial Analysis: Cumulative Year-wise Trend by Trial Recruitment Status
- Figure 7.7 Clinical Trial Analysis: Distribution by Study Design
- Figure 7.8 Clinical Trial Analysis: Distribution by Trial Focus Area
- Figure 7.9 Clinical Trial Analysis: Distribution by Type of Preventive Vaccine (based on Pathogen)
- Figure 7.10 Clinical Trial Analysis: Cumulative Year-wise Trend by Type of Preventive Vaccines
- Figure 7.11 Clinical Trial Analysis: Distribution by Target Disease Indication
- Figure 7.12 Clinical Trial Analysis: Distribution by Patient Enrollment and Target Disease Indication
- Figure 7.13 Clinical Trial Analysis: Distribution by Type of Sponsor / Collaborator
- Figure 7.14 Leading Industry Players: Distribution by Number of Registered Trials
- Figure 7.15 Clinical Trial Analysis: Geographical Distribution by Number of Registered Trials
- Figure 7.16 Clinical Trial Analysis: Geographical Distribution by Enrolled Patient Population
- Figure 7.17 Clinical Trial Analysis: Geographical Distribution by Trial Registration Year and Recruitment Status
- Figure 8.1 Historical Timeline of COVID-19 Vaccine Development
- Figure 8.2 Historical Timeline of Anti-Ebola Virus Vaccine Development
- Figure 8.3 Historical Timeline of Anti-HIV Vaccine Development
- Figure 8.4 Historical Timeline of Anti-Malaria Vaccine Development
- Figure 8.5 Historical Timeline of Anti-Zika Virus Vaccine Development
- Figure 9.1 Funding and Investment Analysis: Cumulative Number of Instances by Year, Since 2015
- Figure 9.2 Funding and Investment Analysis: Cumulative Amount Invested, Since 2015 (USD Million)
- Figure 9.3 Funding and Investment Analysis: Distribution by Type of Funding, Since 2015
- Figure 9.4 Funding and Investment Analysis: Distribution of Amount Invested by Type of Funding, Since 2015 (USD Million)
- Figure 9.5 Funding and Investment Analysis: Distribution of Amount Invested by Type of Vaccine API
- Figure 9.6 Funding and Investment Analysis: Distribution of Amount Invested and Focus Area
- Figure 9.7 Funding and Investment Analysis: Distribution of Amount Invested by Different Type of Investors
- Figure 9.8 Most Active Players: Distribution by Number of Funding Instances
- Figure 9.9 Most Active Investors: Distribution by Number of Funding Instances
- Figure 9.10 Funding and Investment Analysis: Regional Distribution by Number of Funding Instances and Amount Raised (USD Million)
- Figure 9.11 Funding and Investment Analysis: Country-wise Distribution by Total Amount Invested (USD Million)
- Figure 10.1 Overall Preventive Vaccines Market, Till 2030 (USD Billion)
- Figure 10.2 Preventive Vaccines Market, Till 2030: Distribution by Route of Administration (USD Billion)
- Figure 10.3 Preventive Vaccines Market, Till 2030: Distribution by Type of Vaccine (USD Billion)
- Figure 10.4 Preventive Vaccines Market, Till 2030: Distribution by Type of Vaccine API (USD Billion)
- Figure 10.5 Preventive Vaccines Market, Till 2030: Distribution by Target Patient Population (USD Billion)
- Figure 10.6 Preventive Vaccines Market, Till 2030: Distribution by Key Geographical Regions (USD Billion)
- Figure 10.7 Preventive Vaccines Market in North America, Till 2030 (USD Billion)
- Figure 10.8 Preventive Vaccines Market in the US, Till 2030 (USD Billion)
- Figure 10.9 Preventive Vaccines Market in Mexico, Till 2030 (USD Billion)
- Figure 10.10 Preventive Vaccines Market in Canada, Till 2030 (USD Billion)
- Figure 10.11 Preventive Vaccines Market in Europe, Till 2030 (USD Billion)
- Figure 10.12 Preventive Vaccines Market in Spain, Till 2030 (USD Billion)
- Figure 10.13 Preventive Vaccines Market in the UK, Till 2030 (USD Billion)
- Figure 10.14 Preventive Vaccines Market in Italy, Till 2030 (USD Billion)
- Figure 10.15 Preventive Vaccines Market in France, Till 2030 (USD Billion)
- Figure 10.16 Preventive Vaccines Market in Germany, Till 2030 (USD Billion)
- Figure 10.17 Preventive Vaccines Market in Rest of Europe, Till 2030 (USD Billion)
- Figure 10.18 Preventive Vaccines Market in Asia Pacific, Till 2030 (USD Billion)
- Figure 10.19 Preventive Vaccines Market in India, Till 2030 (USD Billion)
- Figure 10.20 Preventive Vaccines Market in China, Till 2030 (USD Billion)
- Figure 10.21 Preventive Vaccines Market in Australia, Till 2030 (USD Billion)
- Figure 10.22 Preventive Vaccines Market in Rest of Asia Pacific, Till 2030 (USD Billion)
- Figure 10.23 Preventive Vaccines Market in Rest of the World, Till 2030 (USD Billion)
- Figure 11.1 Type of Third-Party Service Providers in the Pharmaceutical Industry
- Figure 11.2 Commonly Outsourced Vaccine Development Operations
- Figure 11.3 Key Factors to Consider while Selecting a CMO Partner
- Figure 11.4 Risks and Challenges Associated with Contract Manufacturing
- Figure 11.5 Vaccine CMOs: Distribution by Year of Establishment
- Figure 11.6 Vaccine CMOs: Distribution by Company Size
- Figure 11.7 Vaccine CMOs: Distribution by Scale of Operation
- Figure 11.8 Vaccine CMOs: Distribution by Location of Headquarters (Region and Country-wise)
- Figure 11.9 Vaccine CMOs: Distribution by Location of Manufacturing Facility (Region-wise)
- Figure 11.10 Vaccine CMOs: Distribution by Type of Services Offered
- Figure 11.11 Vaccine CMOs: Distribution by Expression System Used
- Figure 11.12 Vaccine CMOs: Distribution by Type of Vaccine Manufactured
- Figure 11.13 Vaccine CMOs: Distribution by Type of Vaccine Manufactured and Location of Headquarters




