PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1672866

PUBLISHER: Coherent Market Insights | PRODUCT CODE: 1672866
Pediatric Clinical Trials Market, By Phase, By Study Design, By Therapeutic Area, By Geography
Global Pediatric Clinical Trials Market is estimated to be valued at USD 19.26 Bn in 2025 and is expected to reach USD 37.08 Bn by 2032, growing at a compound annual growth rate (CAGR) of 9.8% from 2025 to 2032.
| Report Coverage | Report Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 19.26 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.80% | 2032 Value Projection: | USD 37.08 Bn |
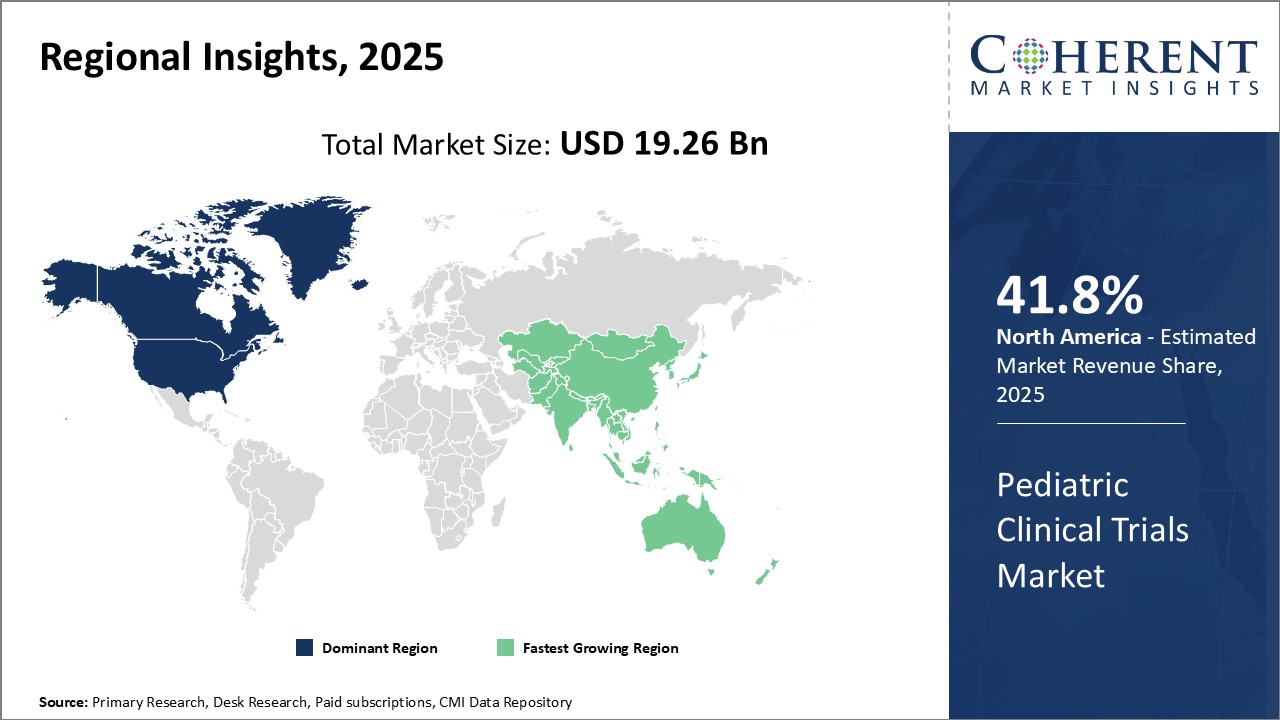
The pediatric clinical trials market is witnessing significant growth in recent times. Growing prevalence of chronic diseases among children and rising demand for new treatment options have boosted the pediatric clinical trials industry. Further, the market has witnessed rising investment from pharmaceutical companies for drug development targeting pediatric population. Growing awareness about rare diseases and various initiatives undertaken by regulatory bodies mandating clinical evaluation of drugs for pediatric use have augmented market opportunities. However, conducting clinical trials for pediatric drugs is more complex and challenging than adult trials due to ethical concerns, age specific drug metabolism, difficulty in recruitment, and lack of consensus over surrogate endpoints. Nevertheless, with emerging novel medical therapies and continuous efforts by players towards developing child-friendly drugs, the pediatric clinical trials market is poised to experience strong growth over the forthcoming years.
Market Dynamics:
The global pediatric clinical trials market growth is driven by the rising incidence of chronic diseases among children, growing demand for new treatment modalities, and initiatives undertaken by regulatory bodies to streamline drug development process. In 2021, as per WHO estimates, over 200 million children suffer from various non-communicable illnesses worldwide. High unmet clinical needs have encouraged pharmaceutical companies to boost investments towards developing specialized treatments for pediatric population. Favorable regulations such as the U.S. Food and Drug Administration's amendments Act 2012 have mandated pre-marketing clinical evaluation of drugs for pediatric use, thereby augmenting market opportunities. However, complexities involved in pediatric clinical research and lack of sufficient surrogate endpoints continue to negatively impact market growth. High costs associated with trials and inadequate number of experienced clinical investigators also impede broader market adoption. Nevertheless, continual collaborations between industry players and research organizations to address issues through novel approaches will support future expansion.
Key Features of the Study:
This report provides in-depth analysis of the global pediatric clinical trials market, and provides market size (US$ Bn) and compound annual growth rate (CAGR%) for the forecast period (2025-2032), considering 2024 as the base year
It elucidates potential revenue opportunities across different segments and explains attractive investment proposition matrices for this market
This study also provides key insights about market drivers, restraints, opportunities, new product launches or approvals, market trends, regional outlook, and competitive strategies adopted by key players
It profiles key players in the global pediatric clinical trials market based on the following parameters - company highlights, products portfolio, key highlights, financial performance, and strategies
Key companies covered as a part of this study include CSL Behring, Sanofi, Takeda Pharmaceutical Company Limited, Orchard Therapeutics plc., Pharming Group N.V., BioCryst Pharmaceuticals, Inc., Ionis Pharmaceuticals, Inc., Attune Pharmaceuticals, Arrowhead Pharmaceuticals, Inc., Adverum Biotechnologies, Inc., KalVista Pharmaceuticals, and CENTOGENE N.V.
Insights from this report would allow marketers and the management authorities of the companies to make informed decisions regarding their future product launches, type up-gradation, market expansion, and marketing tactics
The global pediatric clinical trials market report caters to various stakeholders in this industry including investors, suppliers, product manufacturers, distributors, new entrants, and financial analysts
Stakeholders would have ease in decision-making through various strategy matrices used in analyzing the global pediatric clinical trials market
Market Segmentation
- Phase Insights (Revenue, USD Bn, 2019-2032)
- Phase I
- Phase II
- Phase III
- Phase IV
- Study Design Insights (Revenue, USD Bn, 2019-2032)
- Treatment Studies
- Observational Studies
- Therapeutic Area Insights (Revenue, USD Bn, 2019-2032)
- Respiratory Diseases
- Cardiovascular Diseases
- Neuropsychiatric Conditions
- Oncology
- Diabetes
- Others (Neuropsychiatric Conditions, etc.)
- Regional Insights (Revenue, USD Bn, 2019-2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- Key Players Insights
- CSL Behring
- Sanofi
- Takeda Pharmaceutical Company Limited
- Orchard Therapeutics plc.
- Pharming Group N.V.
- BioCryst Pharmaceuticals, Inc.
- Ionis Pharmaceuticals, Inc.
- Attune Pharmaceuticals
- Arrowhead Pharmaceuticals, Inc.
- Adverum Biotechnologies, Inc.
- KalVista Pharmaceuticals
- CENTOGENE N.V.
Table of Contents
1. Research Objectives and Assumptions
- Research Objectives
- Assumptions
- Abbreviations
2. Market Purview
- Report Description
- Market Definition and Scope
- Executive Summary
- Pediatric Clinical Trials Market, By Phase
- Pediatric Clinical Trials Market, By Study Design
- Pediatric Clinical Trials Market, By Therapeutic Area
- Pediatric Clinical Trials Market, By Region
3. Market Dynamics, Regulations, And Trends Analysis
- Market Dynamics
- Impact Analysis
- Key Highlights
- Regulatory Scenario
- Product Launches/Approvals
- PEST Analysis
- PORTER's Analysis
- Merger and Acquisition Scenario
- Epidemiology
4. Global Pediatric Clinical Trials Market, By Phase, 2020-2032, (USD Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Phase I
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Phase II
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Phase III
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Phase IV
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
5. Global Pediatric Clinical Trials Market, By Study Design, 2020-2032, (USD Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Treatment Studies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Observational Studies
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
6. Pediatric Clinical Trials Market, By Therapeutic Area, 2020-2032, (USD Bn)
- Introduction
- Market Share Analysis, 2025 and 2032 (%)
- Y-o-Y Growth Analysis, 2021 - 2032
- Segment Trends
- Respiratory Diseases
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Cardiovascular Diseases
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Neuropsychiatric Conditions
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Oncology
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Diabetes
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
- Others (Neuropsychiatric Conditions, etc.)
- Introduction
- Market Size and Forecast, and Y-o-Y Growth, 2020-2032, (USD Bn)
7. Global Pediatric Clinical Trials Market, By Region, 2020 - 2032, Value (USD Bn)
- Introduction
- Market Share (%) Analysis, 2025,2028 & 2032, Value (USD Bn)
- Market Y-o-Y Growth Analysis (%), 2021 - 2032, Value (USD Bn)
- Regional Trends
- North America
- Introduction
- Market Size and Forecast, By Phase, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Study Design, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Therapeutic Area, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country, 2020 - 2032, Value (USD Bn)
- U.S.
- Canada
- Latin America
- Introduction
- Market Size and Forecast, By Phase, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Study Design, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Therapeutic Area, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country, 2020 - 2032, Value (USD Bn)
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Introduction
- Market Size and Forecast, By Phase, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Study Design, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Therapeutic Area, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country, 2020 - 2032, Value (USD Bn)
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- Introduction
- Market Size and Forecast, By Phase, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Study Design, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Therapeutic Area, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country, 2020 - 2032, Value (USD Bn)
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- Introduction
- Market Size and Forecast, By Phase, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Study Design, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Therapeutic Area, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country, 2020 - 2032, Value (USD Bn)
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- Introduction
- Market Size and Forecast, By Phase, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Study Design, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Therapeutic Area, 2020 - 2032, Value (USD Bn)
- Market Size and Forecast, By Country/Sub-region, 2020 - 2032, Value (USD Bn)
- South Africa
- North Africa
- Central Africa
8. Competitive Landscape
- CSL Behring
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Sanofi
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Takeda Pharmaceutical Company Limited
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Orchard Therapeutics plc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Pharming Group N.V.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- BioCryst Pharmaceuticals, Inc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Ionis Pharmaceuticals, Inc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Attune Pharmaceuticals
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Arrowhead Pharmaceuticals, Inc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- Adverum Biotechnologies, Inc.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- KalVista Pharmaceuticals
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
- CENTOGENE N.V.
- Company Highlights
- Product Portfolio
- Key Developments
- Financial Performance
- Strategies
9. Analyst Recommendations
- Wheel of Fortune
- Analyst View
- Coherent Opportunity Map
10. References and Research Methodology
- References
- Research Methodology
- About us




