PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1844307

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1844307
Gastrointestinal Stromal Tumor Treatment Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
The Global Gastrointestinal Stromal Tumor Treatment Market was valued at USD 1.5 billion in 2024 and is estimated to grow at a CAGR of 8.3% to reach USD 3.2 billion by 2034.
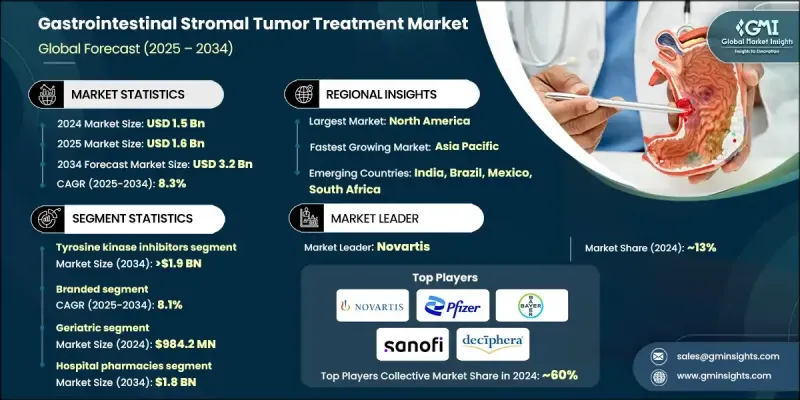
This growth can be attributed to the increasing global prevalence of gastrointestinal cancers and heightened awareness of rare tumor types. Advancements in targeted therapies, particularly tyrosine kinase inhibitors (TKIs), are improving treatment outcomes and survival rates significantly. The rising incidence of metastatic and treatment-resistant GIST cases has further fueled demand for innovative drugs like ripretinib, avapritinib, and regorafenib, which are used to manage disease progression and enhance patient quality of life at various stages of the condition. Treatments for GIST are typically administered orally and are available through hospital pharmacies, retail pharmacies, and online platforms. These therapies include categories such as tyrosine kinase inhibitors, multikinase inhibitors, and combination treatments, all aimed at both primary and metastatic tumors. The market is populated by numerous global pharmaceutical companies that continue to invest heavily in research and development, resulting in the approval of new therapies and expanded treatment indications. The introduction of precision oncology and mutation-specific treatments is reshaping the market, with drugs tailored to specific genetic mutations demonstrating higher efficacy and fewer side effects.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $1.5 Billion |
| Forecast Value | $3.2 Billion |
| CAGR | 8.3% |
In 2024, the tyrosine kinase inhibitors segment held a share of 62.3% in the GIST treatment market, driven by their targeted action and proven clinical effectiveness. As personalized medicine becomes more prevalent, the demand for tyrosine kinase inhibitors has surged. These drugs, particularly imatinib, are considered the standard first-line treatment for metastatic GIST and continue to be preferred due to their high efficacy, as noted by research from the National Institutes of Health (NIH).
The branded drug segment will grow at a CAGR of 8.1% through 2034. Branded therapies are favored due to their demonstrated clinical success, FDA approvals, and consistent inclusion in global treatment guidelines. These treatments target specific genetic mutations like KIT and PDGFRA, which are commonly found in GIST patients. Ongoing investments in research and development are driving the creation of next-generation tyrosine kinase inhibitors (TKIs) and combination therapies designed to overcome drug resistance and improve treatment outcomes.
United States Gastrointestinal Stromal Tumor Treatment Market was valued at USD 561.8 million in 2024, reflecting significant demand. With the advancement of diagnostic imaging and molecular testing, more GIST cases are being accurately detected in the U.S., leading to an increased number of patients seeking treatment. Additionally, supportive FDA regulations and reimbursement policies are facilitating the approval and adoption of both branded and generic treatments, further expanding market opportunities.
Major players in the Gastrointestinal Stromal Tumor Treatment Market include Bayer, Pfizer, Takeda Pharmaceuticals, F. Hoffmann-La Roche, Novartis, Sun Pharma, Shorla Oncology, Glenmark, AngioDynamics, Argon Medical Products, Natco Pharma, and Deciphera Pharma. To strengthen their position in the gastrointestinal stromal tumor treatment market, companies are focusing on several key strategies. This includes heavy investment in research and development (R&D) to bring innovative treatments to market and expand existing product lines. Many companies are prioritizing the development of next-generation tyrosine kinase inhibitors and combination therapies, particularly those targeting resistance mechanisms. Additionally, companies are enhancing their market foothold by forming strategic partnerships with research institutions and other pharmaceutical companies to accelerate drug development and broaden distribution networks. Strengthening brand equity through long-term clinical trial data, FDA approvals, and integration into global treatment guidelines is also a critical focus. Finally, companies are expanding their geographical reach and increasing access to treatments in emerging markets, where the incidence of GIST is rising, thereby maximizing growth potential.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumption and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Drug type trends
- 2.2.3 Type trends
- 2.2.4 Age group trends
- 2.2.5 Distribution channel trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factor affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence gastrointestinal stromal tumors
- 3.2.1.2 Advancing age and genetic predisposition
- 3.2.1.3 Breakthroughs in targeted therapy
- 3.2.1.4 Growing awareness of recurrence and resistance
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Adverse effects and resistance to therapy
- 3.2.2.2 Limited access in low-income regions
- 3.2.3 Market opportunities
- 3.2.3.1 Development of mutation-specific and combination therapies
- 3.2.3.2 Expansion of genomic testing and personalized medicine
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Future market trends
- 3.6 Technological landscape
- 3.6.1 Current technologies
- 3.6.2 Emerging technologies
- 3.7 Pipeline analysis
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Merger and acquisition
- 4.6.2 Partnership and collaboration
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Drug Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Multikinase inhibitors
- 5.3 Tyrosine kinase inhibitors
- 5.4 VEGF inhibitors
Chapter 6 Market Estimates and Forecast, By Type, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Branded
- 6.3 Generics
Chapter 7 Market Estimates and Forecast, By Age Group, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Adults
- 7.3 Geriatric
Chapter 8 Market Estimates and Forecast, By Distribution Channel, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospital pharmacies
- 8.3 Retail pharmacies
- 8.4 Online pharmacies
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Bayer
- 10.2 Deciphera Pharmaceuticals
- 10.3 F. Hoffmann-La Roche
- 10.4 Glenmark
- 10.5 Natco Pharma
- 10.6 Novartis
- 10.7 Pfizer
- 10.8 Sanofi
- 10.9 Shorla Oncology
- 10.10 Sun Pharmaceuticals
- 10.11 Takeda Pharmaceuticals




