PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1844346

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1844346
Vitamin D Testing Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
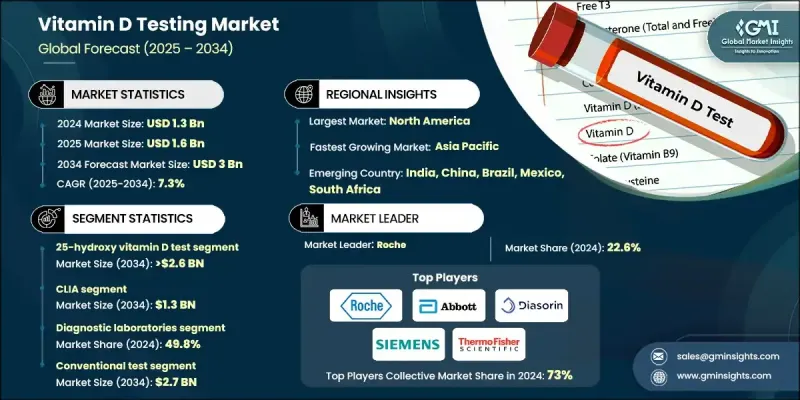
The Global Vitamin D Testing Market was valued at USD 1.3 billion in 2024 and is estimated to grow at a CAGR of 7.3% to reach USD 3 billion by 2034.
The growth is influenced by the increasing prevalence of vitamin D deficiency worldwide, a greater focus on preventive healthcare, and rising demand for diagnostic testing. The continued integration of advanced technologies into healthcare systems is accelerating the adoption of vitamin D diagnostics. With chronic illnesses such as diabetes, autoimmune disorders, cardiovascular diseases, and osteoporosis on the rise, vitamin D testing is becoming a critical part of clinical workflows. Healthcare providers are incorporating these tests into routine diagnostics to manage deficiencies and monitor supplementation outcomes more accurately. Diagnostic labs, payers, and digital health platforms are turning to solutions like LC-MS/MS, ELISA, and chemiluminescence immunoassays for enhanced accuracy and patient monitoring. This is supporting a major shift toward personalized healthcare, where early detection plays a key role. The availability of vitamin D testing in hospitals, clinics, and even at-home setups contributes to increasing accessibility. The expanding focus on chronic disease prevention, maternal and child health, and elderly care programs is further supporting the market's consistent growth trajectory.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $1.3 Billion |
| Forecast Value | $3 Billion |
| CAGR | 7.3% |
The 25-hydroxy vitamin D test segment held 86.2% share in 2024, as it remains the preferred standard for evaluating vitamin D levels in the blood. This segment is projected to reach USD 2.6 billion by 2034, growing at a CAGR of 7.6%. Its use spans multiple clinical applications, including chronic disease management, routine wellness checkups, elderly care, and nutritional assessments. The test is widely adopted to assess deficiency in individuals with limited sunlight exposure, low dietary intake, or metabolic disorders affecting vitamin D absorption. Its role in both population-wide screenings and targeted diagnostics continues to solidify its market dominance.
The CLIA segment held a 43.5% share in 2024 and is expected to reach USD 1.3 billion by 2034. The growing demand for chemiluminescence immunoassay testing is driven by its superior sensitivity and precision over traditional immunoassay methods. CLIA enables accurate quantification of key vitamin D metabolites, which are essential markers in assessing deficiency-related risks. Its adoption is increasing across both centralized labs and point-of-care testing setups due to its reliability, speed, and scalable throughput.
North America Vitamin D Testing Market held a 37.7% share in 2024, driven by advanced healthcare infrastructure, high patient awareness, and proactive screening initiatives. The region is particularly impacted by widespread vitamin D deficiency, especially in areas with reduced sun exposure. As a result, testing is increasingly integrated into routine health evaluations. Physicians regularly recommend these tests for patients with long-term conditions, contributing to increased demand. Widespread insurance coverage, government health campaigns, and growing home-based diagnostics are further accelerating market expansion across the US and Canada.
Key companies shaping the Global Vitamin D Testing Market include Abbott, Thermo Fisher Scientific, Roche, Danaher (Beckman Coulter), Mindray Medical International, NanoSpeed Diagnostics, Siemens, Diasorin, Randox Laboratories, Bio-Rad Laboratories, Tosoh, Qualigen Therapeutics, EUROIMMUN, bioMerieux, and NanoEnTek. To strengthen their foothold, leading players in the vitamin D testing market are implementing a range of strategic initiatives. Many are focused on expanding their diagnostic portfolios by launching highly sensitive, next-generation assay kits that offer faster turnaround times. Companies are also investing in automation and AI-based analytics for enhanced workflow efficiency and data accuracy. Geographic expansion into high-growth regions is being prioritized through distributor partnerships and local manufacturing.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Product trends
- 2.2.3 Technique trends
- 2.2.4 Indication trends
- 2.2.5 Patient trends
- 2.2.6 Test type trends
- 2.2.7 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Component & technology suppliers
- 3.1.2 Diagnostic device & kit manufacturers
- 3.1.3 Value addition at each stage
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing prevalence of vitamin D deficiency disorders
- 3.2.1.2 Rising awareness among consumers
- 3.2.1.3 Rise in public health campaigns and reimbursement policies
- 3.2.1.4 Increasing technological advancements
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of advanced diagnostic methods
- 3.2.3 Market opportunities
- 3.2.3.1 Expansion into point-of-care and home testing
- 3.2.3.2 Growth into emerging markets
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory scenario
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Reimbursement scenario
- 3.6 Technology landscape
- 3.6.1 Current technological trends
- 3.6.2 Emerging technologies
- 3.7 Future market trends
- 3.8 Pricing analysis, 2024
- 3.9 Patent landscape
- 3.9.1 Patent holder analysis
- 3.10 Clinical guidelines & standardization
- 3.11 Investment & funding trends
- 3.12 Epidemiology scenario
- 3.13 Porter's analysis
- 3.14 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.3.1 Global
- 4.3.2 North America
- 4.3.3 Europe
- 4.3.4 Asia Pacific
- 4.3.5 Latin America
- 4.3.6 MEA
- 4.4 Competitive positioning matrix
- 4.5 Competitive analysis of major market players
- 4.6 Key developments
- 4.6.1 Mergers & acquisitions
- 4.6.2 Partnerships & collaborations
- 4.6.3 New service type launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 25-hydroxy vitamin D test
- 5.3 1,25-dihydroxy vitamin D test
Chapter 6 Market Estimates and Forecast, By Technique, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 CLIA
- 6.3 ELISA
- 6.4 LC-MS
- 6.5 Radioimmunoassay
- 6.6 Other techniques
Chapter 7 Market Estimates and Forecast, By Indication, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Vitamin D deficiency
- 7.3 Osteoporosis
- 7.4 Cardiovascular
- 7.5 Rickets
- 7.6 Thyroid disorders
- 7.7 Other indications
Chapter 8 Market Estimates and Forecast, By Patient, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Adult
- 8.3 Pediatric
Chapter 9 Market Estimates and Forecast, By Test Type, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 Conventional test
- 9.3 Point-of-care test
Chapter 10 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 10.1 Key trends
- 10.2 Diagnostic laboratories
- 10.3 Hospitals
- 10.4 Homecare
- 10.5 Other end use
Chapter 11 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 11.1 Key trends
- 11.2 North America
- 11.2.1 U.S.
- 11.2.2 Canada
- 11.3 Europe
- 11.3.1 Germany
- 11.3.2 UK
- 11.3.3 France
- 11.3.4 Spain
- 11.3.5 Italy
- 11.3.6 Netherlands
- 11.4 Asia Pacific
- 11.4.1 China
- 11.4.2 Japan
- 11.4.3 India
- 11.4.4 Australia
- 11.4.5 South Korea
- 11.5 Latin America
- 11.5.1 Brazil
- 11.5.2 Mexico
- 11.5.3 Argentina
- 11.6 Middle East and Africa
- 11.6.1 South Africa
- 11.6.2 Saudi Arabia
- 11.6.3 UAE
Chapter 12 Company Profiles
- 12.1 Abbott
- 12.2 bioMerieux
- 12.3 Bio-Rad Laboratories
- 12.4 Danaher (Beckman Coulter)
- 12.5 Diasorin
- 12.6 EUROIMMUN
- 12.7 Roche
- 12.8 Mindray Medical International
- 12.9 NanoEnTek
- 12.10 NanoSpeed Diagnostics
- 12.11 Qualigen Therapeutics
- 12.12 Randox Laboratories
- 12.13 Siemens
- 12.14 Thermo Fisher Scientific
- 12.15 Tosoh




