PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1858971

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1858971
Gene Therapy Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2024 - 2032
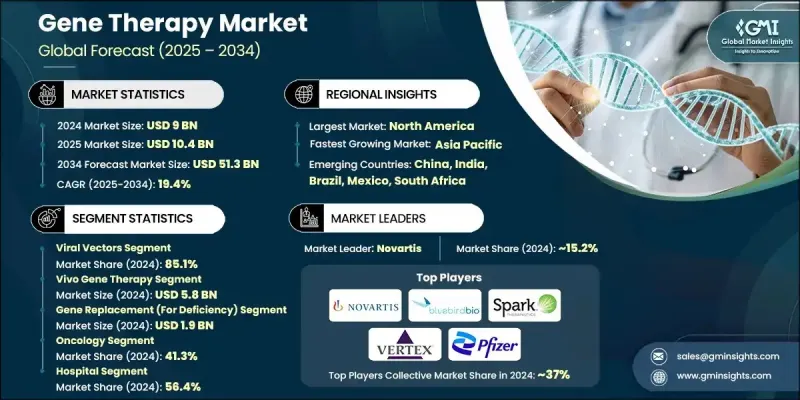
The Global Gene Therapy Market was valued at USD 9 billion in 2024 and is estimated to grow at a CAGR of 19.4% to reach USD 51.3 billion by 2034.
This significant growth trajectory is being fueled by advances in gene delivery systems, rising investments in gene therapy development, the growing prevalence of diseases targeted by gene therapy, such as cancer and rare genetic disorders, and an increasing number of regulatory product approvals. Expanding access to contract manufacturing for gene and cell therapies, along with strategic developments in delivery technology, are further enhancing the market outlook. Regulatory agencies are streamlining approval processes for promising gene therapies, and governments are boosting funding toward innovative genetic medicine platforms. As healthcare systems focus on personalized and targeted treatments, gene therapy is gaining traction for its potential to address complex diseases at the molecular level. Market players are also leveraging digital platforms to share data, accelerate clinical trials, and optimize drug discovery pipelines. Meanwhile, broader integration of precision medicine and bioinformatics is helping gene therapies achieve better treatment efficacy, reduced side effects, and long-term therapeutic benefits. These trends are positioning gene therapy as a disruptive force in the next phase of modern medicine.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $9 Billion |
| Forecast Value | $51.3 Billion |
| CAGR | 19.4% |
In 2024, the viral vectors segment held an 85.1% share, underscoring their continued dominance as the most effective delivery vehicles. These vectors, including adeno-associated viruses and lentiviruses, are widely used due to their superior ability to deliver genetic material accurately and maintain consistent gene expression over time. Their broad clinical application and proven success in approved treatments have reinforced their commercial viability, making them a cornerstone of gene therapy development. Robust manufacturing protocols and scalable production also support their widespread deployment across therapeutic pipelines.
The in vivo gene therapy segment accounted for USD 5.8 billion in 2024, owing to its systemic approach to treating complex and multi-organ diseases. In vivo methods enable direct gene delivery into the body, eliminating the need for cell extraction and reinfusion. This method supports broader therapeutic access and reduces procedural complexity. Advances in non-viral and hybrid delivery systems-such as nanoparticles and lipid-based formulations are further enhancing targeting precision and treatment outcomes.
North America Gene Therapy Market held a 51.2% share in 2024, making it the dominant region for gene therapy innovation and commercialization. This leadership is supported by a robust investment environment, favorable regulatory frameworks, and a sophisticated healthcare infrastructure. With leading biotech firms, prominent academic institutions, and high R&D activity, the region, particularly the U.S., continues to push the boundaries of gene therapy science. Strong public-private collaborations and a deep talent pool are accelerating clinical development and commercial adoption of cutting-edge therapies.
Prominent players in the Global Gene Therapy Market include Sangamo Therapeutics, Amgen, Spark Therapeutics (Roche), Beam Therapeutics, Krystal Biotech, Sarepta Therapeutics, AGTC, Orchard Therapeutics, bluebird bio, Bristol-Myers Squibb Company, Gilead Sciences, Novartis, Helixmith, Pfizer, Intellia Therapeutics, Bayer, UniQure N.V., BioMarin Pharmaceutical, CRISPR Therapeutics, and Audentes Therapeutics (Astellas Pharma). To strengthen their foothold, leading companies in the gene therapy sector are prioritizing strategic mergers and acquisitions to enhance their technology platforms and expand product pipelines. Many firms are entering collaborative R&D partnerships with academic institutions and smaller biotech innovators to access novel therapies and accelerate clinical timelines. Increasing investment in in-house manufacturing capabilities and global supply chain infrastructure ensures faster scaling and regulatory compliance. Companies are also intensifying their focus on securing fast-track, orphan drug, and breakthrough therapy designations to reduce time-to-market.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Vector trends
- 2.2.3 Delivery method trends
- 2.2.4 Gene type trends
- 2.2.5 Indication trends
- 2.2.6 End Use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Advancements in gene editing technologies
- 3.2.1.2 Increasing prevalence of rare genetic and inherited diseases
- 3.2.1.3 Rising investments and collaborations
- 3.2.1.4 Growing awareness and improved genetic diagnostics
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High development and manufacturing costs
- 3.2.2.2 Complexity of gene therapy delivery and potential side effects
- 3.2.3 Market opportunities
- 3.2.3.1 Development of personalized and precision gene therapies
- 3.2.3.2 Expansion in emerging markets with growing healthcare infrastructure
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.1.1 U.S.
- 3.4.1.2 Canada
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.4.4 Latin America
- 3.4.5 Middle East and Africa
- 3.4.1 North America
- 3.5 Technology landscape
- 3.5.1 Current trends
- 3.5.2 Emerging technologies
- 3.6 Pipeline analysis
- 3.7 Future market trends
- 3.8 Porter's analysis
- 3.9 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.2.1 Global
- 4.2.2 North America
- 4.2.3 Europe
- 4.2.4 Asia Pacific
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Merger and acquisition
- 4.6.2 Partnership and collaboration
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Vector, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Viral vectors
- 5.2.1 Retro viral vectors
- 5.2.2 Adeno-associated virus vectors
- 5.2.3 Lentiviral vectors
- 5.2.4 Other viral vectors
- 5.3 Non-viral vectors
- 5.3.1 Plasmid DNA
- 5.3.2 Lipid nanoparticles (LNPs)
- 5.3.3 Oligonucleotides
- 5.3.4 Other non-viral vectors
Chapter 6 Market Estimates and Forecast, By Delivery Method, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 In vivo gene therapy
- 6.3 Ex vivo gene therapy
Chapter 7 Market Estimates and Forecast, By Gene Type, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Antigen-encoding genes
- 7.3 Cytokine-encoding genes
- 7.4 Tumor suppressor genes
- 7.5 Suicide genes
- 7.6 Gene replacement (for deficiency)
- 7.7 Growth factor genes
- 7.8 Receptor-encoding genes
- 7.9 Other gene types
Chapter 8 Market Estimates and Forecast, By Indication, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Oncology
- 8.2.1 Acute Lymphoblastic Leukemia (ALL)
- 8.2.2 Large B-cell lymphoma
- 8.2.3 Head & neck squamous cell carcinoma
- 8.2.4 Melanoma
- 8.3 Neurological disorders
- 8.3.1 Duchenne muscular dystrophy (DMD)
- 8.3.2 Spinal muscular atrophy (SMA)
- 8.4 Hemophilia A & B
- 8.5 Hepatological diseases
- 8.6 Inherited retinal disease
- 8.7 Peripheral arterial disease
- 8.8 Other indications
Chapter 9 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 Hospitals
- 9.3 Specialty clinics and gene therapy centers
- 9.4 Academic and research institutions
- 9.5 Other End uses
Chapter 10 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 10.1 Key trends
- 10.2 North America
- 10.2.1 U.S.
- 10.2.2 Canada
- 10.3 Europe
- 10.3.1 Germany
- 10.3.2 UK
- 10.3.3 France
- 10.3.4 Spain
- 10.3.5 Italy
- 10.3.6 Netherlands
- 10.4 Asia Pacific
- 10.4.1 China
- 10.4.2 India
- 10.4.3 Japan
- 10.4.4 Australia
- 10.4.5 South Korea
- 10.5 Latin America
- 10.5.1 Brazil
- 10.5.2 Mexico
- 10.5.3 Argentina
- 10.6 Middle East and Africa
- 10.6.1 South Africa
- 10.6.2 Saudi Arabia
- 10.6.3 UAE
Chapter 11 Company Profiles
- 11.1 Amgen
- 11.2 Applied Genetic Technologies Corporation (AGTC)
- 11.3 Audentes Therapeutics, Inc. (Astellas Pharma)
- 11.4 Bayer
- 11.5 Beam Therapeutics
- 11.6 BioMarin Pharmaceutical
- 11.7 bluebird bio
- 11.8 Bristol-Myers Squibb Company
- 11.9 CRISPR Therapeutics
- 11.10 Gilead Sciences
- 11.11 Helixmith
- 11.12 Intellia Therapeutics
- 11.13 Krystal Biotech
- 11.14 Novartis
- 11.15 Orchard Therapeutics
- 11.16 Pfizer
- 11.17 Sangamo Therapeutics
- 11.18 Sarepta Therapeutics
- 11.19 Spark Therapeutics (Roche)
- 11.20 UniQure N.V.




