PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1871240

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1871240
U.S. Self-monitoring Blood Glucose Devices Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
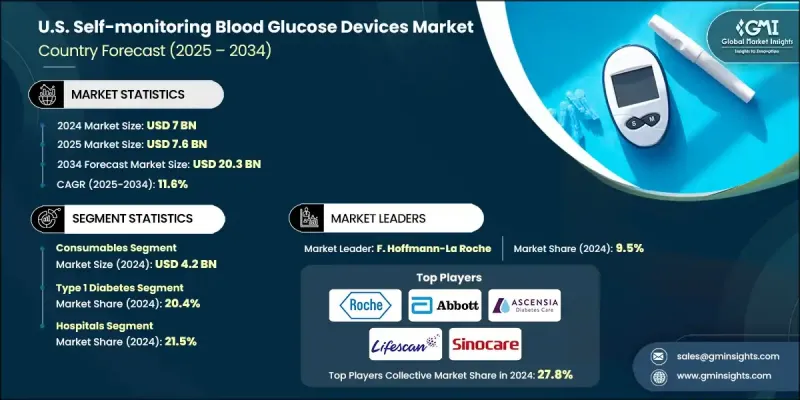
U.S. Self-monitoring Blood Glucose Devices Market was valued at USD 7 Billion in 2024 and is estimated to grow at a CAGR of 11.6% to reach USD 20.3 Billion by 2034.
The growth is driven by the rising prevalence of diabetes, ongoing technological advancements in SMBG devices, and strong policy and reimbursement support. SMBG devices allow individuals with diabetes to monitor their blood sugar levels regularly, enabling better disease management and informed decisions regarding diet, physical activity, and medication. Government programs like Medicare and Medicaid, along with private insurance coverage, are increasingly supporting these advanced devices, lowering out-of-pocket costs for patients. This financial backing encourages healthcare providers to recommend SMBG devices more widely, expanding access to reliable diabetes management tools. SMBG devices, including blood glucose meters, rely on small blood samples collected via lancets and test strips to deliver accurate glucose readings, supporting daily self-care routines.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $7 Billion |
| Forecast Value | $20.3 Billion |
| CAGR | 11.6% |
The consumables segment was valued at USD 4.2 Billion in 2024. Test strips and lancets are essential for daily glucose monitoring and are available over the counter for easy accessibility. Test strips are single-use consumables that collect blood samples for accurate measurement, and their compact design allows patients to monitor glucose levels conveniently at home, work, or while traveling.
The type 1 diabetes segment held a 20.4% share in 2024. Type 1 diabetes is an autoimmune condition that destroys insulin-producing beta cells, resulting in insufficient insulin levels. Regular self-monitoring using SMBG devices is critical for managing blood glucose levels. These devices allow multiple daily checks, helping patients adjust insulin dosage, diet, and exercise, detect fluctuations early, and reduce the risk of severe complications.
The hospitals segment held a 21.5% share in 2024. They act as primary centers for diabetes care, offering diagnosis, treatment initiation, and ongoing monitoring. Equipped with trained professionals and advanced diagnostic technologies, hospitals are central to the adoption of high-tech SMBG solutions. Reimbursement programs and Medicare support further enhance hospitals' ability to integrate advanced glucose monitoring devices into patient care.
Major players in the U.S. Self-monitoring Blood Glucose Devices Market include Abbott Laboratories, AgaMatrix, All Medicus, Arkray, Ascensia Diabetes Care Holdings, B. Braun Melsungen, Bionime Corporation, DarioHealth, F. Hoffmann-La Roche, LifeScan, Nova Biomedical, Omnis Health, Sanofi, Sinocare, and Ypsomed Holding. Key strategies employed by companies in the U.S. Self-monitoring Blood Glucose Devices Market include continuous investment in R&D to enhance accuracy, usability, and connectivity of devices, launching innovative products that integrate AI or digital health platforms, and forming partnerships with hospitals, pharmacies, and insurers to expand distribution channels. Companies also focus on patient education programs, subscription-based consumables models, and strategic acquisitions to diversify offerings and strengthen market presence.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 360° synopsis
- 2.2 Key market trends
- 2.2.1 Product trends
- 2.2.2 Applications
- 2.2.3 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Value addition at each stage
- 3.1.3 Factor affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Growing prevalence of diabetes across U.S.
- 3.2.1.2 Shift toward value-based and remote care
- 3.2.1.3 Increased healthcare spending and accessibility
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of advanced SMBG devices and accessories
- 3.2.2.2 Stringent regulatory scenario
- 3.2.3 Market opportunities
- 3.2.3.1 Integration of AI and smart ecosystems
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological advancements
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Supply chain and distribution analysis
- 3.7 Reimbursement scenario
- 3.8 Pricing analysis, 2024
- 3.9 Future market trends
- 3.10 Gap analysis
- 3.11 Porter's analysis
- 3.12 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Strategic dashboard
- 4.7 Key developments
- 4.7.1 Mergers and acquisitions
- 4.7.2 Partnerships and collaborations
- 4.7.3 New product launches
- 4.7.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Blood glucose monitoring devices
- 5.3 Consumables
- 5.3.1 Testing strips
- 5.3.2 Lancets
Chapter 6 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Type 1 diabetes
- 6.3 Type 2 diabetes
- 6.4 Gestational diabetes
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospital
- 7.3 Ambulatory surgical centers
- 7.4 Diagnostic centers
- 7.5 Homecare
- 7.6 Other end use
Chapter 8 Company Profiles
- 8.1 Abbott Laboratories
- 8.2 AgaMatrix
- 8.3 All Medicus
- 8.4 Arkray
- 8.5 Ascensia Diabetes Care Holdings
- 8.6 B. Braun Melsungen
- 8.7 Bionime Corporation
- 8.8 DarioHealth
- 8.9 F. Hoffmann-La Roche
- 8.10 LifeScan
- 8.11 Nova Biomedical
- 8.12 Omnis Health
- 8.13 Sanofi
- 8.14 Sinocare
- 8.15 Ypsomed Holding




