PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1876652

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1876652
Barth Syndrome Treatment Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
The Global Barth Syndrome Treatment Market was valued at USD 157.1 million in 2024 and is estimated to grow at a CAGR of 11.2% to reach USD 442.3 million by 2034.
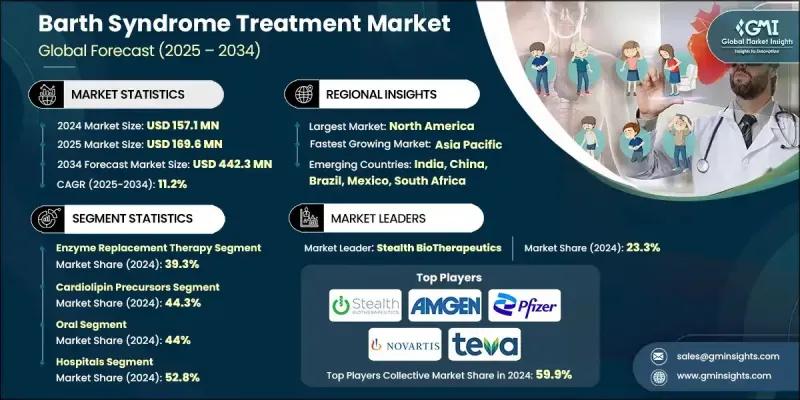
The market continues to expand as the number of diagnosed cases rises, awareness improves among clinicians and families, and significant advancements occur in gene-based and enzyme-focused therapies. The industry supports biotechnology developers, pharmaceutical companies, research institutions, and healthcare providers by offering specialized solutions for genetic research, therapeutic development, and patient care. Treatment options span gene therapy, enzyme replacement therapy, and supportive care aimed at improving cardiac and muscular symptoms while enhancing overall quality of life. Progress in gene therapy has strengthened market momentum, with emerging methods designed to correct TAZ gene mutations that drive the condition, potentially delivering long-term therapeutic benefits. Enzyme replacement therapies are evolving rapidly as they work to restore mitochondrial function and address the underlying biochemical defects. Supportive care, including pharmacologic management of cardiomyopathy and hematologic complications, continues to play a vital role in improving survival outcomes and sustaining market growth.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $157.1 Million |
| Forecast Value | $442.3 Million |
| CAGR | 11.2% |
The enzyme replacement therapy segment held a 39.3% share in 2024 owing to its direct role in addressing tafazzin deficiency, which significantly improves mitochondrial activity and enhances patient outcomes. Its targeted mechanism, which replenishes the missing enzyme to support cardiac and muscular function, has positioned it as a leading treatment option for individuals with Barth syndrome.
The cardiolipin precursors segment held a 44.3% share in 2024 and is expected to reach USD 208.8 million during 2025-2034. These treatments command substantial usage because they help restore mitochondrial membrane stability, compensating for impaired cardiolipin remodeling caused by TAZ gene mutations, which disrupt energy production and cellular performance.
North America Barth Syndrome Treatment Market held a 35.3% share in 2024, supported by a highly developed healthcare infrastructure and widespread access to diagnostic testing for rare genetic disorders. Early detection enabled by next-generation sequencing and other advanced laboratory tools contributes to prompt therapeutic intervention and better long-term outcomes for patients.
Key companies active in the Global Barth Syndrome Treatment Market include Stealth BioTherapeutics, TransCellular Therapeutics (TCT), Abbott, Nutricia, Amneal Pharmaceuticals, Boehringer Ingelheim, Amgen, Teva, Novartis, Scenic Biotech, Pfizer, and B. Braun. Companies in the Barth Syndrome Treatment Market pursue several strategic approaches to reinforce their presence. Many focus on expanding research programs centered on gene therapy and enzyme replacement therapy to develop treatments capable of addressing the root cause of the disorder. Collaborations between pharmaceutical firms, academic research teams, and biotechnology innovators help accelerate clinical development and regulatory progress. Firms also invest in advanced manufacturing technologies to support consistent production of specialized therapies.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definitions
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Therapeutic approaches trends
- 2.2.3 Drug class trends
- 2.2.4 Route of administration trends
- 2.2.5 End Use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Rising awareness & improved diagnostics
- 3.2.1.2 Regulatory incentives for orphan/rare diseases
- 3.2.1.3 Advances in therapeutic science
- 3.2.1.4 Growing healthcare investment in rare diseases
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 Extremely small patient population
- 3.2.2.2 Clinical trial & recruitment challenges
- 3.2.3 Market opportunities
- 3.2.3.1 Development of curative/novel therapies
- 3.2.3.2 Patient registry & real-world evidence initiatives
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.4.1 North America
- 3.4.2 Europe
- 3.4.3 Asia Pacific
- 3.5 Technology landscape
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies and their impacts
- 3.6 Future market trends
- 3.6.1 Regulatory milestone accelerating market entry
- 3.6.2 Increased R&D and investment activity
- 3.6.3 Enhanced clinical validation for mitochondrial therapies
- 3.7 Pricing analysis
- 3.8 Investment and funding landscape
- 3.9 Pipeline landscape
- 3.10 Porter's analysis
- 3.11 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.3.1 Global
- 4.3.2 North America
- 4.3.3 Europe
- 4.3.4 Asia Pacific
- 4.3.5 LAMEA
- 4.4 Competitive positioning matrix
- 4.5 Competitive analysis of major market players
- 4.6 Key developments
- 4.6.1 Mergers & acquisitions
- 4.6.2 Partnerships & collaborations
- 4.6.3 Expansion plans
Chapter 5 Market Estimates and Forecast, By Therapeutic Approaches, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Enzyme replacement therapy
- 5.3 Gene therapy
- 5.4 Supportive care
- 5.5 Stem cell therapy
- 5.6 Other therapeutic approaches
Chapter 6 Market Estimates and Forecast, By Drug Class, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Cardiolipin precursors
- 6.3 Antioxidants
- 6.4 Immunomodulators
- 6.5 Dietary supplements
- 6.6 Antibiotics
- 6.7 Other drug classes
Chapter 7 Market Estimates and Forecast, By Route of Administration, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Oral
- 7.3 Intravenous
- 7.4 Subcutaneous
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Hospitals
- 8.3 Specialty clinics
- 8.4 Other End Use
Chapter 9 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 9.1 Key trends
- 9.2 North America
- 9.2.1 U.S.
- 9.2.2 Canada
- 9.3 Europe
- 9.3.1 Germany
- 9.3.2 UK
- 9.3.3 France
- 9.3.4 Spain
- 9.3.5 Italy
- 9.3.6 Netherlands
- 9.4 Asia Pacific
- 9.4.1 China
- 9.4.2 Japan
- 9.4.3 India
- 9.4.4 Australia
- 9.4.5 South Korea
- 9.5 Latin America
- 9.5.1 Brazil
- 9.5.2 Mexico
- 9.5.3 Argentina
- 9.6 Middle East and Africa
- 9.6.1 South Africa
- 9.6.2 Saudi Arabia
- 9.6.3 UAE
Chapter 10 Company Profiles
- 10.1 Abbott
- 10.2 Amgen
- 10.3 Amneal Pharmaceuticals
- 10.4 B. Braun
- 10.5 Boehringer Ingelheim
- 10.6 Novartis
- 10.7 Nutricia
- 10.8 Pfizer
- 10.9 Scenic Biotech
- 10.10 Stealth BioTherapeutics
- 10.11 Teva
- 10.12 TransCellular Therapeutics (TCT)




