PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1437490

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1437490
France Insulin Drugs And Delivery Devices - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029)
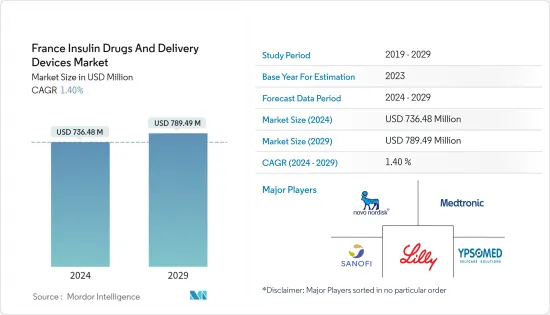
The France Insulin Drugs And Delivery Devices Market size is estimated at USD 736.48 million in 2024, and is expected to reach USD 789.49 million by 2029, growing at a CAGR of 1.40% during the forecast period (2024-2029).
One of the most significant risk factors for a severe course of COVID-19 is diabetes mellitus. This risk is believed to be influenced by several variables that are frequently present in diabetes mellitus, such as advanced age, a proinflammatory and hypercoagulable condition, hyperglycemia, and underlying comorbidities (hypertension, cardiovascular disease, chronic kidney disease, and obesity). Diabetes was quickly recognized as a risk factor for bad results during the COVID-19 pandemic. That's why managing or delaying cases of type 2 diabetes became more important than ever before. Several studies have confirmed that chronic diseases like diabetes are associated with adverse outcomes in COVID-19 patients.
Diabetes is associated with many health complications. Comparing the population with and without diabetes, those with diabetes have a 300% increased risk of being hospitalized and, thus, incur more healthcare expenses compared to non-diabetic people. People with diabetes face a higher chance of experiencing serious complications from COVID-19. In general, people with diabetes are more likely to experience severe symptoms and complications when infected with a virus. Diabetes and high glucose levels are associated with increased complications, respiratory failure, and mortality in hospitalized patients with coronavirus.
Diabetes has the highest prevalence among all chronic conditions covered 100% by France's statutory health insurance (SHI), and the number of covered patients has doubled in the past 10 years. France is one of the major countries in the Europe region. The pandemic also highlighted opportunities for continuing and expanding innovations in the delivery of diabetes care, through virtual consultations between healthcare providers and people with diabetes, and the use of diabetes technology. Crisis management has created unprecedented interest in remote care from both patients and providers and removed many long-standing regulatory barriers. Thus, the COVID-19 outbreak increased the insulin market's growth.
France Insulin Drugs And Delivery Devices Market Trends
Growing Diabetes Population and Research Studies in France
In France, the diabetes population is expected to increase with a CAGR of about 2.9% over the forecast period.
Moreover, according to IDF 2021 report, 61 million individuals in the Europe Region and 537 million people worldwide have diabetes; by 2045, this number will increase to 69 million. According to a survey by the EU Commission, the number of persons in France who have chronic diabetes is the greatest, and one in 10 people in France has diabetes. The prevalence of diabetes is significant in France, although the rules' target of 80% compliance with advised healthcare consultations isn't always met. To promote proactive therapy adjustment and decrease therapeutic inertia, new techniques that focus on patient and physician education and information are necessary to lessen the burden of complications and hospitalizations associated with diabetes.
In November 2022, the EU Commission and member states demonstrated political commitment. They set ambitious targets to 'reduce inequalities between EU citizens and improve the care and quality of life of people with diabetes.' The experts emphasized the importance of ensuring effective, accessible, and uninterrupted treatments to reduce the risk of developing complications. The resolution's authors also emphasized the importance of data collection standardization. They highlighted the role of the Commission's 'Healthier Together' initiative, launched in December 2021, which aims to prevent, manage, and treat non-communicable diseases (NCDs). The resolution calls on member states to develop, implement and monitor national diabetes plans and strategies with' comparable milestones and targets' in recognizing inequalities in public health strategies and care and access to healthy and sustainable food across member states.
In France, there is a centralized health care system their all the reimbursement decisions are taken at a national level and then applied to all the countries. The French health benefit basket plays an important role; diabetes is one of the 30 chronic diseases covered 100% by statutory health insurance (SHI) under the ALD scheme (affections de longue duree). For those not covered under ALD, the patient pays a share of the official health care tariff, which varies depending on the category of goods and care.
Therefore, owing to the increasing diabetes prevalence, the studied market is anticipated to grow over the analysis period.
The insulin pumps segment is expected to witness the highest growth rate over the forecast period
The insulin pumps segment holds a major market share in the insulin delivery devices market this year. It is expected to grow with a CAGR of about 4.3% over the forecast period because of the increasing technological advancement and its preference over other traditional methods due to continuous insulin administration.
An insulin pump is a small battery-operated electronic device about the size of a pager or small mobile phone. The rapid-acting insulin is delivered via an infusion set which is inserted subcutaneously. The device delivers insulin in two ways, basal and bolus. An insulin pump is worn 24 hours a day but can be taken off for up to two hours when required. Data can be uploaded from insulin pumps via web-based software. The data relating to glucose concentrations and insulin delivery can be reviewed by the health professional with the patient. Randomized controlled trials have reported improved glycemic control using insulin pump therapy compared to multiple daily injections. These include reductions in blood glucose, hypoglycemia, lower glycated hemoglobin, lower insulin requirements, and improved quality of life.
Type-1 diabetic patients must check their blood glucose levels regularly, monitor their blood glucose levels, and adjust the insulin dosing to maintain optimum glucose levels. The use of continuous subcutaneous insulin infusion and continuous glucose monitoring systems has improved patient care and quality of life and is widely used in the ambulatory setting. Increasingly, this technology is also being used in the hospital setting. There are pump models with remote controls enabling parents of young children to either suspend or bolus insulin from a distance when the child is playing or eating. The insulin infusion pumps reduce the large swings in blood glucose levels, induce less pain, and deliver more accurately when compared to injections. These advantages of insulin pumps over the traditional delivery system are expected to boost the market.
France Insulin Drugs And Delivery Devices Industry Overview
The France Insulin Drugs and Delivery Devices Market is moderately consolidated, with few significant and generic players. Mergers and acquisitions between the players recently have helped the companies strengthen their market presence. Eli Lilly and Boehringer Ingelheim have an alliance in developing and commercializing Basaglar (Insulin Glargine). Additionally, the players in the recent past helped the companies strengthen their market presence; for example, Novo Nordisk collaborated with Ypsomed to provide better insulin therapy solutions.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.3 Market Restraints
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Bargaining Power of Suppliers
- 4.4.2 Bargaining Power of Consumers
- 4.4.3 Threat of New Entrants
- 4.4.4 Threat of Substitute Products and Services
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION
- 5.1 Drug
- 5.1.1 Basal or Long-acting Insulins
- 5.1.1.1 Lantus (Insulin Glargine)
- 5.1.1.2 Levemir (Insulin Detemir)
- 5.1.1.3 Toujeo (Insulin Glargine)
- 5.1.1.4 Tresiba (Insulin Degludec)
- 5.1.1.5 Basaglar (Insulin Glargine)
- 5.1.2 Bolus or Fast-acting Insulins
- 5.1.2.1 NovoRapid/Novolog (Insulin aspart)
- 5.1.2.2 Humalog (Insulin lispro)
- 5.1.2.3 Apidra (Insulin glulisine)
- 5.1.2.4 FIASP (Insulin aspart)
- 5.1.2.5 Admelog (Insulin lispro Sanofi)
- 5.1.3 Traditional Human Insulins
- 5.1.3.1 Novolin/Mixtard/Actrapid/Insulatard
- 5.1.3.2 Humulin
- 5.1.3.3 Insuman
- 5.1.4 Combination Insulins
- 5.1.4.1 NovoMix (Biphasic Insulin aspart)
- 5.1.4.2 Ryzodeg (Insulin degludec and Insulin aspart)
- 5.1.4.3 Xultophy (Insulin degludec and Liraglutide)
- 5.1.4.4 Soliqua/Suliqua (Insulin glargine and Lixisenatide)
- 5.1.5 Biosimilar Insulins
- 5.1.5.1 Insulin Glargine Biosimilars
- 5.1.5.2 Human Insulin Biosimilars
- 5.1.1 Basal or Long-acting Insulins
- 5.2 Device
- 5.2.1 Insulin Pumps
- 5.2.1.1 Insulin Pump Devices
- 5.2.1.2 Insulin Pump Reservoirs
- 5.2.1.3 Insulin Infusion sets
- 5.2.2 Insulin Pens
- 5.2.2.1 Cartridges in reusable pens
- 5.2.2.2 Disposable insulin pens
- 5.2.3 Insulin Syringes
- 5.2.4 Insulin Jet Injectors
- 5.2.1 Insulin Pumps
6 MARKET INDICATORS
- 6.1 Type-1 Diabetes Population
- 6.2 Type-2 Diabetes Population
7 COMPETITIVE LANDSCAPE
- 7.1 COMPANY PROFILES
- 7.1.1 Novo Nordisk
- 7.1.2 Sanofi
- 7.1.3 Eli Lilly
- 7.1.4 Biocon
- 7.1.5 Medtronic
- 7.1.6 Ypsomed
- 7.1.7 Becton Dickinson
- 7.1.7.1 Other Key Players
- 7.2 COMPANY SHARE ANALYSIS
- 7.2.1 Insulin Drugs
- 7.2.1.1 Novo Nordisk
- 7.2.1.2 Sanofi
- 7.2.1.3 Eli Lilly
- 7.2.2 Insulin Delivery Devices
- 7.2.2.1 Medtronic
- 7.2.2.2 Ypsomed
- 7.2.2.3 Becton Dickinson
- 7.2.1 Insulin Drugs
8 MARKET OPPORTUNITIES AND FUTURE TRENDS




