PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1408609

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1408609
Small Molecules Contract Development and Manufacturing Organization - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts 2024 - 2029
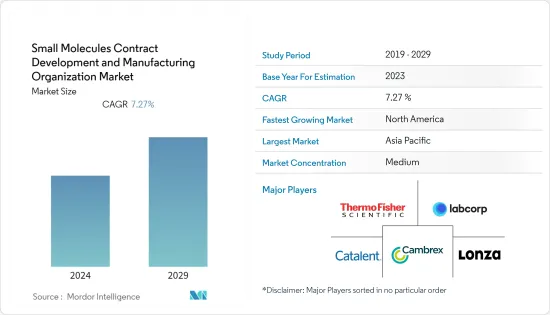
The small molecules contract development and manufacturing organization (CDMO) market was valued at USD 180.52 billion in 2024. It is expected to reach USD 273.7 billion by the end of the forecast period, registering a CAGR of 7.27%.
The COVID-19 pandemic significantly impacted the growth of the small molecule CDMO market. Research groups worldwide implemented various strategies to identify small-molecule drugs to treat COVID-19. For instance, according to the article published in the Nature Journal in April 2022, the SARS-CoV2 3CL protease was identified as a critical drug target for small molecule COVID-19 therapy, given its efficacy and essentiality in the viral maturation and replication cycle. The emergence of such active small molecules for the treatment of COVID-19 significantly contributed to the market's growth. Moreover, pharmaceutical companies were continuously engaged in drug development and collaborated with various CDMOs to manufacture COVID-19 therapeutic products. For instance, in March 2023, Samsung Biologics (CDMO) signed on to manufacture COVID-19 vaccines for Moderna. Hence, the small molecule contract development and manufacturing organization market witnessed growth during the pandemic.
The major factors driving the market's growth are increasing pharmaceutical and biotechnology R&D investment, growing demand for small molecules, and rising incidence of chronic diseases.
Small molecule drugs have more demand since they are way more affordable than biological drugs. They have continuously helped to advance medicine and address unmet medical needs. One of the primary factors responsible for the rise in demand for small-molecule medicines is an increase in regulatory approvals. For instance, in December 2022, the FDA's CDER approved 20 new small molecule drugs, or 63% of the total 32 new drugs approved thus far in 2022. Thus, increasing regulatory approval for small molecules is boosting the demand for small molecule development and manufacturing services and is contributing to the market's growth.
The increasing number of small molecules in the pipeline on expedited regulatory pathways being developed by small and emerging pharma companies and a trend toward greater complexity of chemical structures are contributing to the market's growth. Small molecules are likely to continue to represent the majority of prescribed drugs in the coming years, driving the growth of the CDMO market. Accelerating advancement in research and technology is generating opportunities for biopharmaceutical companies to develop innovative small-molecule drugs. Hence, considering the dominance of this type of medicine on the market, around one-third of the CMOs offer commercial-scale small molecule API as well as advanced intermediates manufacturing, compared to 20% that provide biologic API manufacturing.
Besides, the increasing complexity associated with small molecules also contributes to the market's growth. According to Lonza, the complexity of chemical synthesis has almost doubled from eight chemical steps to an average of 14 in 2021. The development of complex generics in an era of increasing costs and increased focus on global developments and manufacturing operations requires a higher level of expertise. It demands more in-depth planning and a deep understanding of the regulatory, quality, and clinical aspects of small molecule development to bring these drugs to the market. Hence, this is likely to create demand for small molecule CDMO services, thereby contributing to the market's growth over the forecast period.
Furthermore, strategic initiatives taken by market players, such as expansion of services portfolios, mergers, collaboration, and partnerships, are likely to increase demand for these services. This is anticipated to boost the demand for small molecules CDMO services over the forecast period. For instance:
Key Highlights
- In March 2023, Catalent and Grunenthal announced their successful formulation design and clinical-phase manufacturing collaboration for an orally dosed small molecule in Grunenthal's pipeline.
- In September 2022, Cambrex announced the completion of the first phase of its USD 30 million investment in its small molecule active pharmaceutical ingredient (API) manufacturing facility in High Point, North Carolina.
- In June 2022, Lonza inaugurated a new clinical phase development and manufacturing facility in its small molecules site in Bend, Oregon. It produces bioavailability-enhancing spray-dried dispersion (SDD) finished dosage forms and drug product intermediates.
Hence, increasing demand for small molecule therapeutics, growing R&D investment for the same, and increasing complexity associated with the manufacturing of small molecules are expected to boost the market over the forecast period. However, stringent government regulations and compliance issues with outsourcing are anticipated to restrain the market over the forecast period.
Small Molecules Contract Development And Manufacturing Organization Market Trends
Oncology Segment is Expected to Witness a Major Share in the Market Studied over the Forecast Period
Small molecule drugs have been widely used in cancer treatment procedures for decades for their various benefits, such as high efficacy and selectivity, convenience, ability to penetrate cancerous cells and deliver medication, and wide range of target receptors. Some of the most common small molecule drug compounds used as cancer therapeutics include kinase inhibitors, epigenetic regulatory proteins, DNA damage repair enzymes, and proteasomes.
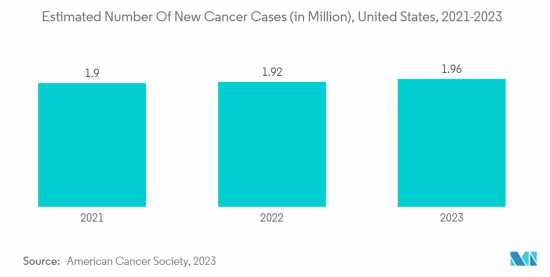
The increasing prevalence of cancer is fueling the demand for advanced and effective therapeutics, leading to new investments by companies and other stakeholders, like governments, for the identification, testing, and development of novel cancer therapeutics. For instance, according to the Canadian Cancer Society's report of 2022, an estimated 233,900 people were diagnosed with cancer in 2022. Similarly, according to the Cancer Australia data published in May 2022, approximately 14,529 lung cancer cases were diagnosed in Australia in the same year, which is up from 13,810 lung cancer cases in 2021. Thus, the growing burden of cancer is expected to create a demand for drug development and manufacturing services, which is likely to contribute to the segment's growth.
Moreover, strategic activities by market players, such as expansion of services, collaboration, and signing agreements with other companies, are expected to boost the market's growth. For instance, in March 2023, CatSci Ltd partnered with AGC Pharma Chemicals, a global small molecule CDMO. This enables CatSci's customers to harness AGC's expertise in GMP manufacturing from kilos to tonnes to support clinical phase projects, including cancer and infectious diseases. Similarly, in April 2022, Societal CDMO Inc. announced that it was awarded a new manufacturing and packaging task order agreement by the National Cancer Institute's Division of Cancer Treatment and Diagnosis (DCTD).
Hence, the growing burden of cancer, increasing investment for the development of small molecules cancer drugs, and strategic collaboration by the market players are expected to boost the segment's growth.
North America is Expected to Hold a Significant Market Share Over the Forecast Period
In North America, the small molecules CDMO market is expected to grow due to factors such as established research facilities and high investment in R&D. Furthermore, the strong foothold of key market players and rising grants by the National Institute of Health for developing novel therapeutics in the country are also contributing to the market's growth.
The increasing research and development and strong pipeline of small molecule drugs are expected to boost the demand for CDMO services, contributing to the market's growth. For instance, in January 2022, Sanofi signed a research collaboration and license agreement with Exscientia for the development of up to 15 novel small molecule candidates in the oncology and immunology field. Furthermore, under the agreement, Exscientia and Sanofi work together to identify and choose target projects by utilizing Exscientia's platform for personalized medicine.
The growing trend of outsourcing among pharmaceutical companies is expected to drive the market's growth during the forecast period. For instance, in March 2022, Acanthus Research Inc. announced the launch of Acanthus Pharma Services Inc., a rapidly expanding CDMO organization focusing on providing services to the pharmaceutical and biotechnology industry. The company provides organic synthesis services such as specialty chemicals and organic chemistry services. Similarly, in October 2023, Ampio Pharmaceuticals Inc. announced that it selected Ascendia Pharmaceuticals Inc. to provide services to support the clinical development of OA-201, a novel therapeutic for the treatment of symptomatic osteoarthritis pain.
Hence, growing R&D investment for the development of small molecules and strategic activities by the market players is expected to boost the market over the forecast period.
Small Molecules Contract Development And Manufacturing Organization Industry Overview
The small molecules contract development and manufacturing organization market is consolidated in nature and is highly competitive. The players are engaging in strategic activities such as service expansion, collaboration, partnerships, and mergers and acquisitions. Some of the key players in the small molecules CDMO market are Catalent Inc., Lonza, Cambrex Corporation, Thermo Fisher Scientific Inc., Labcorp Drug Development, and IQVIA Inc.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definitions
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Demand for Small Molecule Drugs
- 4.2.2 Growing Burden of Chronic Diseases
- 4.2.3 Increasing Pharmaceutical R&D Investments
- 4.3 Market Restraints
- 4.3.1 Stringent Government Regulations
- 4.3.2 Compliance Issues with Outsourcing
- 4.4 Porter's Five Forces Analysis
- 4.4.1 Bargaining Power of Suppliers
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Threat of New Entrants
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD)
- 5.1 By Product
- 5.1.1 Small Molecule API
- 5.1.2 Small Molecule Drug Product
- 5.1.2.1 Oral Solid dose
- 5.1.2.2 Semi-solid Dose
- 5.1.2.3 Liquid Dose
- 5.1.2.4 Other Small Molecule Drug Products
- 5.2 By Stage
- 5.2.1 Preclinical
- 5.2.2 Clinical
- 5.2.2.1 Phase I
- 5.2.2.2 Phase II
- 5.2.2.3 Phase III
- 5.2.2.4 Phase IV
- 5.2.3 Commercial
- 5.3 By End User
- 5.3.1 Pharmaceutical and Biotechnology Companies
- 5.3.2 Research Institutes and Academics
- 5.4 By Therapeutic Area
- 5.4.1 Cardiovascular Diseases
- 5.4.2 Oncology
- 5.4.3 Respiratory disorders
- 5.4.4 Neurology
- 5.4.5 Metabolic disorders
- 5.4.6 Infectious diseases
- 5.4.7 Other Therapeutic Areas
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 United Kingdom
- 5.5.2.2 Germany
- 5.5.2.3 France
- 5.5.2.4 Spain
- 5.5.2.5 Italy
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 India
- 5.5.3.2 Japan
- 5.5.3.3 China
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East and Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of the Middle East and Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Eurofins Scientific
- 6.1.2 Cambrex Corporation
- 6.1.3 Catalent
- 6.1.4 Thermo Fisher Scientific Inc.
- 6.1.5 Jubilant Pharmova Limited
- 6.1.6 Lonza Group Ltd
- 6.1.7 Wuxi AppTec
- 6.1.8 Syngene International Limited
- 6.1.9 Almac Group
- 6.1.10 Piramal Pharma Solutions
- 6.1.11 Recipharm AB
- 6.1.12 Labcorp Drug Development
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




