PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1842689

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1842689
Hepatitis Therapeutics - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The hepatitis therapeutics market size reached USD 17.44 billion in 2025 and is forecast to advance to USD 20.83 billion by 2030, reflecting a 3.61% CAGR.
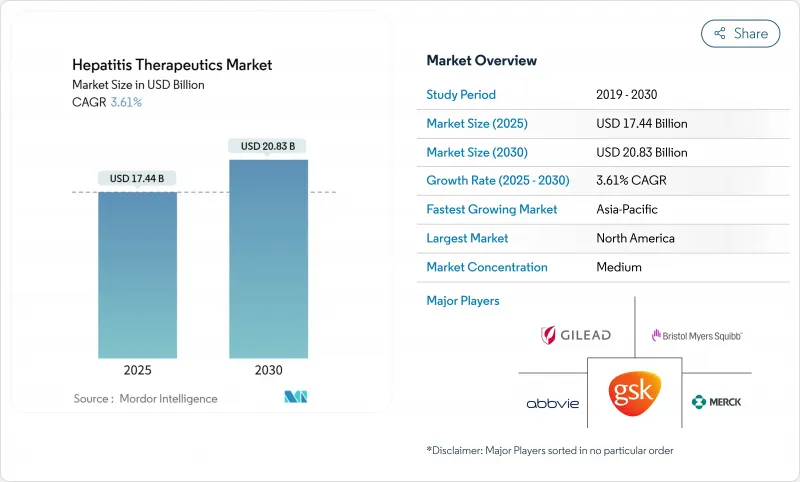
This growth trajectory hides a structural pivot from suppressive regimens toward functional-cure combinations that attack viral replication, immune evasion, and host-factor interactions in parallel. Subscription-style procurement in Louisiana, which treated more than 11,000 residents at negotiated prices, demonstrates how value-based models can unlock latent demand while containing budget impact. In parallel, the WHO's 2024 report that viral hepatitis now causes 1.3 million annual deaths, second only to tuberculosis, has accelerated elimination roadmaps and lifted diagnosis targets worldwide. North America continues to anchor 40.59% of global revenue. Yet, Asia-Pacific delivers the fastest regional expansion at 4.64% CAGR thanks to China and India's combined hepatitis B and C caseload exceeding 35 million.
Global Hepatitis Therapeutics Market Trends and Insights
High Prevalence of Viral Hepatitis
More than 304 million people live with chronic hepatitis B or C, a pool that sustains long-run demand independent of economic fluctuations. China and India contribute over 35 million cases, positioning Asia-Pacific as the principal growth engine for the hepatitis therapeutics market. Only 13% of chronic hepatitis B infections are diagnosed, and a mere 3% receive antiviral therapy, underscoring how underdiagnosis continues to inflate unmet need. Mortality is rising even where incidence is falling, revealing gaps in screening and linkage-to-care pathways that governments now frame as public-health emergencies. These epidemiological realities fortify long-term volume growth for companies able to scale affordable, decentralized treatment models.
Rapid Launch of Pan-Genotypic DAA Regimens
Eight-week pan-genotypic combinations have condensed treatment courses and removed the need for genotype testing in most settings. AbbVie's MAVYRET received FDA clearance as the first 8-week therapy for acute hepatitis C, recording a 96% cure rate while slashing clinic visits. Atea Pharmaceuticals' bemnifosbuvir-ruzasvir combo posted a 98% sustained virologic response in Phase 2 and is moving into Phase 3 in 2025. Shorter regimens reduce loss-to-follow-up and ease scale-up in rural clinics, enhancing the hepatitis therapeutics market's penetration potential.
Uneven Reimbursement in Emerging Economies
Only 52% of low- and middle-income countries reimburse DAA therapy, with many imposing specialist-only prescribing rules that narrow access channels. Development Impact Bond pilots in Cameroon achieved 96% cure rates but remain localized, indicating innovative finance alone cannot overcome systemic funding gaps. These disparities create a two-tier hepatitis therapeutics market where ability to pay, not disease burden, dictates uptake, limiting upside in regions with the largest patient pools.
Other drivers and restraints analyzed in the detailed report include:
- Government-Led Awareness Campaigns & Vaccination Drives
- Expanding Reimbursement in High-Income Countries
- Stringent Regulatory Approval Timelines
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Hepatitis C retained 77.12% revenue in 2024, underpinned by pan-genotypic regimens exceeding 95% cure rates. Nevertheless, hepatitis B is projected to outpace all other indications at 5.02% CAGR through 2030 as RNA interference, monoclonal antibodies, and capsid-assembly modulators converge in multi-agent protocols aimed at surface-antigen loss. The hepatitis therapeutics market size for hepatitis B therapies is on a steady growth trajectory, with multiple Phase 2 and 3 trials targeting HBsAg clearance through novel mechanisms, reinforcing the strategic shift underway.
As functional-cure expectations rise, manufacturers are reprioritizing pipeline capital toward B-specific platforms. Clinical programs such as Arbutus Biopharma's imdusiran, which delivered a 50% functional cure rate in Phase 2a, highlight the commercial upside available to successful entrants. Hepatitis D, while niche, offers a precedent for accelerated adoption once first-in-class therapies clear regulatory hurdles.
NS5A inhibitors accounted for 35.03% revenue in 2024, anchored by sofosbuvir/velpatasvir's 98% cure performance across genotypes. Yet monoclonal antibodies are forecast to grow 4.38% CAGR, the fastest among classes, propelled by candidates such as GIGA-2339, which combines more than 1,000 anti-HBs antibodies and exhibits 2,000-fold greater potency than current options. Multi-class cocktails pairing nucleo(t)ide reverse-transcriptase inhibitors with novel agents form the centerpiece of future filings.
For late-entry competitors, platform breadth outweighs single-asset strength. Firms able to assemble NS5A, siRNA, and antibody assets under one roof can tailor regimens to genotype, fibrosis stage, and prior therapy resistance, deepening share of wallet across the hepatitis therapeutics market.
The Hepatitis Therapeutics Market Report is Segmented by Disease Type (Hepatitis A, Hepatitis B, and More), by Drug Class (Interferons, Monoclonal Antibodies, and More), Route of Administration (Oral and Injectable), Distribution Channel (Offline and Online), End-User (Hospitals, Specialty Clinics, and More) and Geography (North America, Europe, Asia-Pacific, and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America held 40.59% of revenue in 2024 and advances at 3.45% CAGR to 2030. Subscription contracts, value-based reimbursement, and generous Medicaid carve-outs underpin demand and insulate volumes from list-price deflation. The proposed federal carve-out program would replicate Louisiana's flat-fee model nationally, establishing predictable multi-year procurement windows that favor companies with broad portfolios. Canada's centralized purchasing and Mexico's emergent generic hubs complement the region's volume stability.
Europe posts a steady 3.23% CAGR, powered by coordinated elimination plans and early adoption of novel modalities such as bulevirtide for hepatitis D. Risk-sharing payment contracts in Italy and Germany tie reimbursement to sustained virologic response, ensuring therapy access while safeguarding budgets. Regional reference pricing pressures margins, but centralized approvals expedite multi-country launches, allowing quicker payback on development costs.
Asia-Pacific is the growth pacesetter at 4.64% CAGR. The hepatitis therapeutics market size for the region is projected to expand quickly as China rolls out national screening and as India integrates antivirals into public insurance. China documented a 31.54% fall in hepatitis C incidence but a 28.60% mortality increase, underscoring diagnostic lag. Japan and South Korea furnish high-value demand through aggressive screening of aging populations, while Australia's outcomes-based DAA deal has become a case study for neighboring health ministries. Governments across Southeast Asia are earmarking special budgets to meet the WHO 2030 elimination milestone, further amplifying regional momentum.
- Abbvie
- Alnylam Pharmaceuticals
- Arbutus Biopharma Corporation
- Assembly Biosciences, Inc.
- Atea Pharmaceuticals
- Bristol-Myers Squibb
- Cipla
- Dr. Reddy's Laboratories
- Enanta Pharmaceuticals
- Roche
- Gilead Sciences
- Grifols
- GlaxoSmithKline
- Johnson & Johnson
- Merck
- Novartis
- Sun Pharmaceuticals Industries
- Viatris
- Vir Biotechnology, Inc.
- Zydus Lifesciences Ltd
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 High prevalence of viral hepatitis
- 4.2.2 Rapid launch of pan-genotypic DAA regimens
- 4.2.3 Government-led awareness campaigns
- 4.2.4 Expanding reimbursement in high-income countries
- 4.2.5 Rise of pay-for-cure value-based contracts
- 4.2.6 AI-enabled drug-repurposing pipelines
- 4.3 Market Restraints
- 4.3.1 Uneven reimbursement in emerging economies
- 4.3.2 Stringent regulatory approval timelines
- 4.3.3 Stigma-driven under-diagnosis in PWID populations
- 4.3.4 API supply-chain concentration risk in LMICs
- 4.4 Value Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technological Outlook
- 4.7 Porter's Five Forces Analysis
- 4.7.1 Threat of New Entrants
- 4.7.2 Bargaining Power of Suppliers
- 4.7.3 Bargaining Power of Buyers
- 4.7.4 Threat of Substitutes
- 4.7.5 Competitive Rivalry
5 Market Size & Growth Forecasts (Value)
- 5.1 By Disease Type
- 5.1.1 Hepatitis A
- 5.1.2 Hepatitis B
- 5.1.3 Hepatitis C
- 5.1.4 Hepatitis D
- 5.1.5 Other Types
- 5.2 By Drug Class
- 5.2.1 Interferons
- 5.2.2 Monoclonal Antibodies
- 5.2.3 NS5A Inhibitors
- 5.2.4 Nucleotide Analog RT Inhibitors
- 5.2.5 Nucleotide Analog NS5B Inhibitors
- 5.2.6 Multi-class Combinations
- 5.2.7 Other Drug Classes
- 5.3 By Route of Administration
- 5.3.1 Oral
- 5.3.2 Injectable
- 5.4 By Distribution Channel
- 5.4.1 Offline
- 5.4.2 Online
- 5.5 By End-User
- 5.5.1 Hospitals
- 5.5.2 Specialty Clinics
- 5.5.3 Home-care Settings
- 5.5.4 Other End-Users
- 5.6 By Geography
- 5.6.1 North America
- 5.6.1.1 United States
- 5.6.1.2 Canada
- 5.6.1.3 Mexico
- 5.6.2 Europe
- 5.6.2.1 Germany
- 5.6.2.2 United Kingdom
- 5.6.2.3 France
- 5.6.2.4 Italy
- 5.6.2.5 Spain
- 5.6.2.6 Rest of Europe
- 5.6.3 Asia-Pacific
- 5.6.3.1 China
- 5.6.3.2 India
- 5.6.3.3 Japan
- 5.6.3.4 Australia
- 5.6.3.5 South Korea
- 5.6.3.6 Rest of Asia-Pacific
- 5.6.4 Middle East and Africa
- 5.6.4.1 GCC
- 5.6.4.2 South Africa
- 5.6.4.3 Rest of Middle East and Africa
- 5.6.5 South America
- 5.6.5.1 Brazil
- 5.6.5.2 Argentina
- 5.6.5.3 Rest of South America
- 5.6.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Competitive Benchmarking
- 6.3 Market Share Analysis
- 6.4 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.4.1 AbbVie Inc.
- 6.4.2 Alnylam Pharmaceuticals, Inc.
- 6.4.3 Arbutus Biopharma Corporation
- 6.4.4 Assembly Biosciences, Inc.
- 6.4.5 Atea Pharmaceuticals
- 6.4.6 Bristol Myers Squibb
- 6.4.7 Cipla Ltd
- 6.4.8 Dr. Reddy's Laboratories
- 6.4.9 Enanta Pharmaceuticals
- 6.4.10 F. Hoffmann-La Roche
- 6.4.11 Gilead Sciences
- 6.4.12 Grifols, S.A.
- 6.4.13 GSK plc
- 6.4.14 Johnson & Johnson
- 6.4.15 Merck & Co., Inc.
- 6.4.16 Novartis AG
- 6.4.17 Sun Pharmaceutical Industries Limited
- 6.4.18 Viatris Inc.
- 6.4.19 Vir Biotechnology, Inc.
- 6.4.20 Zydus Lifesciences Ltd
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-need Assessment




