PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1844656

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1844656
Asthma And COPD Drugs - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The Asthma and COPD Drugs Market size reached USD 27.58 billion in 2025 and is projected to expand to USD 35.36 billion by 2030, posting a 5.09% CAGR over the forecast period.
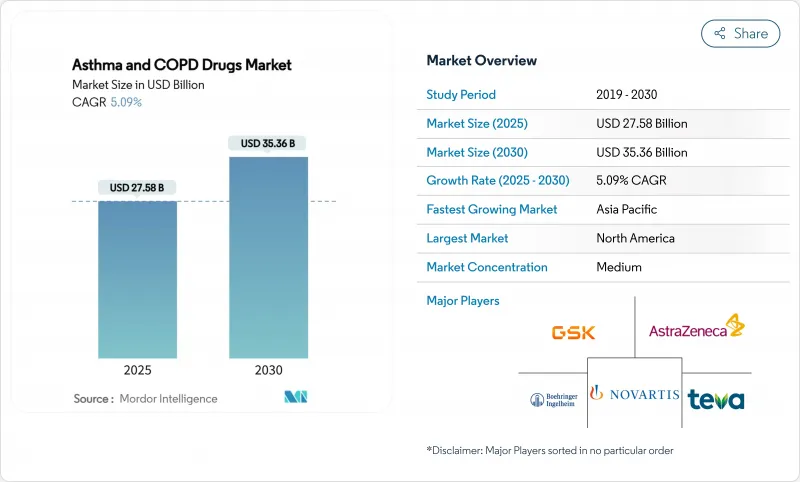
Demand for precision medicine, breakthrough biologic approvals, smart-inhaler roll-outs and steady reimbursement expansion in emerging economies underpin momentum in the Asthma and COPD Drugs Market. Competitive intensity is rising as first-in-class biologics for chronic obstructive pulmonary disease (COPD) reshape treatment algorithms, while fixed-dose triple inhalers and once-daily regimens address adherence shortfalls. Digital inhaler sensors supplying real-time data to clinicians are moving care toward anticipatory management, and payers in Asia-Pacific and Latin America are widening access to high-value respiratory therapies. Alongside these forces, escalating air-pollution exposure in large urban centers continues to enlarge the addressable patient pool for the Asthma and COPD Drugs Market.
Global Asthma And COPD Drugs Market Trends and Insights
Surge in Biologics and Targeted Therapy Approvals for Severe Uncontrolled Asthma
The United States Food and Drug Administration (FDA) cleared dupilumab for COPD in September 2024 after trials showed 30-34% fewer exacerbations, sparking a cascade of biologic launches. GSK's mepolizumab won COPD approval in May 2025, and AstraZeneca's benralizumab is in late-stage trials aimed at eosinophilic inflammation. Developers are now pursuing long-dosing-interval antibodies such as GSK's depemokimab, which delivers six-month coverage, and broad-spectrum agents such as tezepelumab that lower asthma exacerbations by up to 71% irrespective of phenotype . Collectively, these biologics pivot treatment away from symptom control toward disease modification, positioning the Asthma and COPD Drugs Market for sustainable value growth.
Expansion of Healthcare Expenditure and Reimbursement for Respiratory Therapies in Emerging Markets
Governments in Asia-Pacific are instituting reference-pricing frameworks and pharmacoeconomic reviews that reward confirmed clinical benefit while containing spend. China projects a USD 3,296 billion COPD burden by 2039, prompting reimbursement expansion for biologics and infrastructure investment. Australia has piloted financially-based patient-access schemes for high-budget-impact respiratory drugs. Such initiatives support reliable market entry for innovation, cushioning price-sensitive populations and lifting the Asthma and COPD Drugs Market.
Intensifying Generic Competition Following Key Inhaler Patent Expiries
Patents on major inhalers such as Flovent HFA lapse in July 2025, exposing brands to generic attack. Complex device patents and rigorous bio-equivalence demands limit the number of approved generics, yet erosion pressures are unavoidable, trimming near-term value in portions of the Asthma and COPD Drugs Market.
Other drivers and restraints analyzed in the detailed report include:
- Growing Adoption of Fixed-Dose Combination & Once-Daily Inhalers to Improve Patient Compliance
- Advancements in Inhaler Technologies Enhancing Drug Delivery
- High Treatment Costs of Biologics Limiting Access in Cost-Sensitive Regions
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Bronchodilators retained a 38.44% hold on the Asthma and COPD Drugs Market share in 2024, but monoclonal antibodies chart the swiftest climb at a 6.81% CAGR to 2030. Generic short-acting Beta 2-agonists remain the rescue mainstay; however, AstraZeneca's albuterol-budesonide combo introduces anti-inflammatory rescue in a single device, challenging long-established patterns. Long-acting agents are progressively packaged into triple combinations, while phosphodiesterase-4 inhibitors gain relevance through Verona Pharma's dual pathway Ohtuvayre. Antibody developers are now outpacing small-molecule launches, delivering sustained symptom control and disease-modification prospects that lift average revenue per patient.
In the second half of the decade, the ability of biologics to tap overlapping inflammatory cascades is expected to sustain a leadership premium, steering formulary positioning toward broad-spectrum agents. The Asthma and COPD Drugs Market size for monoclonal antibodies is therefore projected to narrow the legacy bronchodilator gap despite higher injection-route complexity. Differentiation by dosing interval and phenotype-agnostic efficacy should drive brand loyalty, while exposure to upcoming biosimilars remains a medium-term consideration.
Inhaled drugs controlled 68.45% of the Asthma and COPD Drugs Market size during 2024 and remain the frontline modality thanks to localized delivery and rapid bronchodilation. Patent expiries on inhaler brands and ecological pressure to replace hydrofluoroalkane propellants are prompting device innovation with near-zero warming potential. Smart-inhaler connectivity embeds analytics into routine care, nudging adherence upward.
Injectable and other parenteral formats log the strongest trajectory at 6.71% CAGR through 2030, propelled by dupilumab, mepolizumab, and tezepelumab uptake. Four-week to six-month subcutaneous schedules ease clinic visits, mitigating historic aversion to injections and elevating share in the Asthma and COPD Drugs Market. Oral agents preserve a niche for anti-leukotrienes and emerging PDE-4 inhibitors, while early-stage inhaled biologics may further fragment delivery-route dynamics beyond 2030.
The Asthma and COPD Drugs Market Report is Segmented by Drug Class (Bronchodilators, and More), Route of Administration (Inhaled, and More), Indication (Asthma, Chronic Obstructive Pulmonary Disease), Prescription Type (Prescription Rx, Over-The-Counter OTC), Distribution Channel (Hospital Pharmacies, and More), and Geography (North America, and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America contributed 36.56% of Asthma and COPD Drugs Market revenue in 2024 on the back of advanced insurance coverage and an innovation-friendly FDA that granted first-in-class COPD biologic status to dupilumab. High inhaler prices, often exceeding USD 600 per month for the uninsured, are intensifying policy debates on patent reform and generic incentives. Canada benefits from provincial reimbursement but negotiates aggressively on biologic pricing, whereas Mexico's expanding private healthcare segment opens new demand corridors.
Europe maintains strong market stature, with centralized EMA approvals accelerating multi-country launches. Environmental regulation is nudging manufacturers toward climate-neutral propellants, a shift embraced in product pipelines. Health-technology-assessment bodies in Germany, the United Kingdom and France scrutinize cost-effectiveness, compelling outcome-based pricing models. Southern Europe shows slower biologic uptake due to budget ceilings, yet long-term savings from exacerbation prevention underpin gradual listing decisions.
Asia-Pacific is the fastest-growing bloc, advancing at 6.43% CAGR between 2025-2030. China's projected COPD economic burden of USD 3,296 billion by 2039 is pushing authorities to expand specialty clinics and reimburse novel modalities. Japan's super-aged population drives premium product uptake, while India leverages domestic manufacturing for cost-efficient generics without forfeiting biologic imports for severe cases. Southeast Asia's urban pollution, linked to 8.1 million global deaths in 2021, is heightening awareness and screening, thereby enlarging the Asthma and COPD Drugs Market.
- AstraZeneca
- Boehringer Ingelheim
- GlaxoSmithKline
- Novartis
- Roche
- Pfizer
- Sanofi
- Merck
- Teva Pharmaceutical Industries
- Chiesi Farmaceutici
- Azurity Pharmaceuticals
- Grifols
- Viatris
- Cipla
- Orion
- Regeneron Pharmaceuticals
- Amgen
- Sun Pharmaceuticals Industries
- Theravance Biopharma Inc.
- Verona Pharma PLC
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Surge in Biologics and Targeted Therapy Approvals for Severe Uncontrolled Asthma
- 4.2.2 Expansion of Healthcare Expenditure and Reimbursement for Respiratory Therapies in Emerging Markets
- 4.2.3 Growing Adoption of Fixed-Dose Combination & Once-Daily Inhalers to Improve Patient Compliance
- 4.2.4 Advancements in Inhaler Technologies Enhancing Drug Delivery
- 4.2.5 Rising Prevalence of Asthma and COPD around the World
- 4.2.6 Increasing Air Pollution in Densely Populated Countries
- 4.3 Market Restraints
- 4.3.1 Intensifying Generic Competition Following Key Inhaler Patent Expiries
- 4.3.2 Stringent Regulatory & Safety Requirements Prolonging Approval Timeline
- 4.3.3 High Treatment Costs of Biologics Limiting Access in Cost-Sensitive Regions
- 4.3.4 Long-Term Corticosteroid & Long-Acting Beta-Agonists (LABA) Safety Concerns Impacting Prescriber Confidence
- 4.4 Value / Supply-Chain Analysis
- 4.5 Regulatory Outlook
- 4.6 Technological Outlook
- 4.7 Porter's Five Forces
- 4.7.1 Threat of New Entrants
- 4.7.2 Bargaining Power of Buyers
- 4.7.3 Bargaining Power of Suppliers
- 4.7.4 Threat of Substitutes
- 4.7.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Drug Class
- 5.1.1 Bronchodilators
- 5.1.1.1 Short-acting Beta 2 Agonists
- 5.1.1.2 Long-acting Beta 2 Agonists
- 5.1.1.3 Anticholinergic Agents
- 5.1.2 Anti-inflammatory Drugs
- 5.1.2.1 Oral & Inhaled Corticosteroids
- 5.1.2.2 Anti-leukotrienes
- 5.1.2.3 Phosphodiesterase-4 Inhibitors
- 5.1.2.4 Other Anti-inflammatory Drugs
- 5.1.3 Monoclonal Antibodies
- 5.1.4 Combination Drugs
- 5.1.1 Bronchodilators
- 5.2 By Route of Administration
- 5.2.1 Inhaled
- 5.2.2 Oral
- 5.2.3 Injectable / Parenteral
- 5.3 By Indication
- 5.3.1 Asthma
- 5.3.2 Chronic Obstructive Pulmonary Disease
- 5.4 By Prescription Type
- 5.4.1 Prescription (Rx)
- 5.4.2 Over-the-Counter (OTC)
- 5.5 By Distribution Channel
- 5.5.1 Hospital Pharmacies
- 5.5.2 Retail Pharmacies
- 5.5.3 Online Pharmacies
- 5.6 By Geography
- 5.6.1 North America
- 5.6.1.1 United States
- 5.6.1.2 Canada
- 5.6.1.3 Mexico
- 5.6.2 Europe
- 5.6.2.1 Germany
- 5.6.2.2 United Kingdom
- 5.6.2.3 France
- 5.6.2.4 Italy
- 5.6.2.5 Spain
- 5.6.2.6 Rest of Europe
- 5.6.3 Asia-Pacific
- 5.6.3.1 China
- 5.6.3.2 Japan
- 5.6.3.3 India
- 5.6.3.4 South Korea
- 5.6.3.5 Australia
- 5.6.3.6 Rest of Asia-Pacific
- 5.6.4 Middle East and Africa
- 5.6.4.1 GCC
- 5.6.4.2 South Africa
- 5.6.4.3 Rest of Middle East and Africa
- 5.6.5 South America
- 5.6.5.1 Brazil
- 5.6.5.2 Argentina
- 5.6.5.3 Rest of South America
- 5.6.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level Overview, Core Business Segments, Financials, Headcount, Key Information, Market Rank, Market Share, Products and Services, and Analysis of Recent Developments)
- 6.3.1 AstraZeneca PLC
- 6.3.2 Boehringer Ingelheim GmbH
- 6.3.3 GlaxoSmithKline PLC
- 6.3.4 Novartis AG
- 6.3.5 F. Hoffmann-La Roche Ltd
- 6.3.6 Pfizer Inc.
- 6.3.7 Sanofi SA
- 6.3.8 Merck & Co., Inc.
- 6.3.9 Teva Pharmaceutical Industries Ltd
- 6.3.10 Chiesi Farmaceutici SpA
- 6.3.11 Azurity Pharmaceuticals, Inc.
- 6.3.12 Grifols SA
- 6.3.13 Viatris Inc.
- 6.3.14 Cipla Ltd
- 6.3.15 Orion Corporation
- 6.3.16 Regeneron Pharmaceuticals Inc.
- 6.3.17 Amgen Inc.
- 6.3.18 Sun Pharmaceutical Industries Limited
- 6.3.19 Theravance Biopharma Inc.
- 6.3.20 Verona Pharma PLC
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-Need Assessment




