PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1850388

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1850388
Bioreactor - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The global bioreactor market size stood at USD 5.68 billion in 2025 and is projected to reach USD 8.17 billion by 2030, advancing at a 7.10% CAGR during 2025-2030.
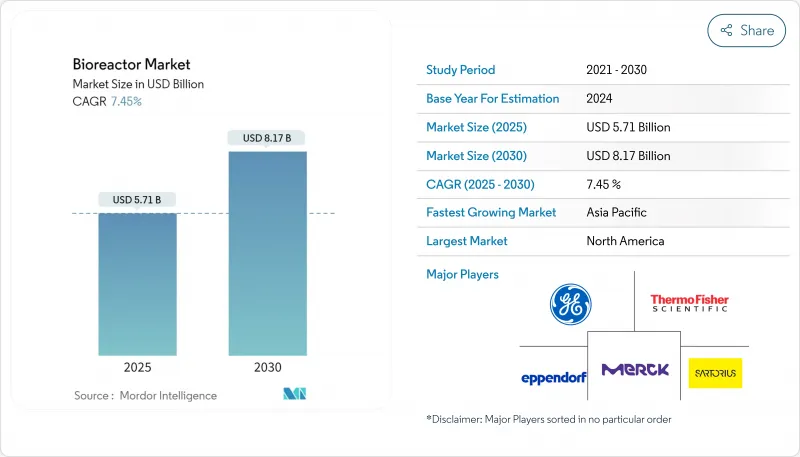
Sustained demand for complex biologics, a rapidly expanding pipeline of cell- and gene-therapy candidates, and steady improvements in process-control technology underpin this growth trajectory. Suppliers that balance flexibility with scale continue to gain ground because stainless-steel, single-use, and hybrid systems each solve distinct production challenges. Automated control is now the default configuration for new installations, and AI-driven analytics are moving from proof-of-concept trials to routine manufacturing, improving batch consistency and reducing downtime. Regional dynamics are also shifting: Asia's accelerated build-out of cGMP capacity is reshaping global supply chains, while North America maintains the largest installed base and remains the launchpad for next-generation technologies.
Global Bioreactor Market Trends and Insights
Rapid Capacity Expansion for Cell & Gene Therapy Manufacturing
Developers of cell- and gene-based products have quickly exceeded the capabilities of legacy monoclonal-antibody equipment. Novel high-density platforms such as Corning's Ascent Fixed-Bed Reactor supply extensive surface area for adherent cultures while preserving a compact footprint, easing viral-vector bottlenecks. CDMOs value these systems because they scale up rapidly without full facility overhauls. As regulators emphasize modality-specific process understanding, purpose-built bioreactors have shifted from optional upgrades to essential infrastructure, adding measurable uplift to the bioreactor market.
Shift Toward Modular & Closed-System Facilities in Emerging Markets
Manufacturers in Asia, Latin America, and parts of Africa are bypassing conventional clean-room builds by installing prefabricated, closed-system suites that integrate single-use assemblies and skid-mounted utilities. These plants reduce capital expenditure, compress validation timelines, and allow faster entry into global supply chains. Their portability also lets CDMOs relocate or expand with minimal disruption, a benefit proven during pandemic-related shortages. The distributed model lowers freight risk and brings production closer to patients, broadening the bioreactor market footprint across more locations.
Sterilization-Integrity Failures in Large-Volume Single-Use Bioreactors
Scaling disposable bags beyond 2,000 L increases stress on seams and film interfaces, raising contamination risk late in production runs. Inconsistent film extrusion and challenges in validating gamma-irradiation efficacy across thicker assemblies remain unresolved. Many producers hedge by pairing single-use seed trains with stainless-steel production tanks, trading some flexibility for sterility assurance. While suppliers are refining test protocols and upgrading materials, the added cost erodes portions of the single-use value proposition, tempering bioreactor market growth.
Other drivers and restraints analyzed in the detailed report include:
- Rise of Continuous Bioprocessing Platforms
- Government Incentives for Vaccine Biomanufacturing in Middle East & Africa
- Global Shortage of High-Quality Single-Use Plastic Resin
For complete list of drivers and restraints, kindly check the Table Of Contents.
List of Companies Covered in this Report:
- Sartorius
- Thermo Fisher Scientific
- Merck
- Danaher
- Eppendorf
- GE Healthcare
- Getinge
- Infors HT
- Bioengineering
- Solaris Biotech
- PBS Biotech Inc.
- Esco Lifesciences
- ABEC
- Cellexus International Ltd.
- Distek Inc.
- Pierre Gurin Technologies
- Bionet Innovative Technologies
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Rapid Capacity Expansion for Cell & Gene-Therapy Manufacturing
- 4.2.2 Shift Toward Modular & Closed-System Facilities in Emerging Markets
- 4.2.3 Rise of Continuous Bioprocessing Platforms
- 4.2.4 Government Incentives for Vaccine Biomanufacturing in Middle East & Africa
- 4.2.5 Collaborative CDMO Outsourcing Driving Single-Use Uptake in Latin America
- 4.2.6 Technological Advancements in Bioreactors
- 4.3 Market Restraints
- 4.3.1 Sterilisation-Integrity Failures in Large-Volume SUBs
- 4.3.2 Global Shortage of High-Quality Single-Use Plastic Resin
- 4.3.3 CapEx Constraints for Stainless-Steel Retrofits in Legacy Plants
- 4.3.4 Complex Regulatory Validation for Hybrid Configurations
- 4.4 Regulatory Outlook
- 4.5 Porter's Five Forces Analysis
- 4.5.1 Threat of New Entrants
- 4.5.2 Bargaining Power of Buyers/Consumers
- 4.5.3 Bargaining Power of Suppliers
- 4.5.4 Threat of Substitute Products
- 4.5.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Type
- 5.1.1 Glass
- 5.1.2 Stainless Steel
- 5.1.3 Single-Use
- 5.2 By Usage
- 5.2.1 Lab-scale Production
- 5.2.2 Pilot-scale Production
- 5.2.3 Full-scale Production
- 5.3 By Scale
- 5.3.1 5 L - 20 L
- 5.3.2 20 L - 200 L
- 5.3.3 200 L - 1,500 L
- 5.3.4 Above 1,500 L
- 5.4 By Control Type
- 5.4.1 Manual
- 5.4.2 Automated (MFCs)
- 5.5 By Bioprocess
- 5.5.1 Batch
- 5.5.2 Fed-batch
- 5.5.3 Continuous
- 5.6 By Application
- 5.6.1 Pharmaceutical & Biopharmaceutical Manufacturing
- 5.6.2 Cell & Gene Therapy
- 5.6.3 Industrial Biotechnology (Biofuels, Enzymes)
- 5.7 By End User
- 5.7.1 Biopharma & Pharma Companies
- 5.7.2 Contract Development & Manufacturing Organisations (CDMOs)
- 5.7.3 Other End Users
- 5.8 By Geography
- 5.8.1 North America
- 5.8.1.1 United States
- 5.8.1.2 Canada
- 5.8.1.3 Mexico
- 5.8.2 Europe
- 5.8.2.1 Germany
- 5.8.2.2 United Kingdom
- 5.8.2.3 France
- 5.8.2.4 Italy
- 5.8.2.5 Spain
- 5.8.2.6 Rest of Europe
- 5.8.3 Asia-Pacific
- 5.8.3.1 China
- 5.8.3.2 Japan
- 5.8.3.3 India
- 5.8.3.4 South Korea
- 5.8.3.5 Australia
- 5.8.3.6 Rest of Asia-Pacific
- 5.8.4 Middle-East and Africa
- 5.8.4.1 GCC
- 5.8.4.2 South Africa
- 5.8.4.3 Rest of Middle East and Africa
- 5.8.5 South America
- 5.8.5.1 Brazil
- 5.8.5.2 Argentina
- 5.8.5.3 Rest of South America
- 5.8.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Share Analysis
- 6.4 Company Profiles (includes Global level Overview, Market level overview, Core Business Segments, Financials, Headcount, Key Information, Market Rank, Market Share, Products and Services, and analysis of Recent Developments)
- 6.4.1 Sartorius AG
- 6.4.2 Thermo Fisher Scientific Inc.
- 6.4.3 Merck KGaA
- 6.4.4 Danaher
- 6.4.5 Eppendorf AG
- 6.4.6 GE Healthcare
- 6.4.7 Getinge AB
- 6.4.8 Infors HT
- 6.4.9 Bioengineering AG
- 6.4.10 Solaris Biotech
- 6.4.11 PBS Biotech Inc.
- 6.4.12 Esco Lifesciences Group
- 6.4.13 ABEC Inc.
- 6.4.14 Cellexus International Ltd.
- 6.4.15 Distek Inc.
- 6.4.16 Pierre Gurin Technologies
- 6.4.17 Bionet Innovative Technologies
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-need Assessment




