PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851108

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851108
Botulinum Toxin - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The botulinum toxin market generated USD 9.77 billion in 2025 and is forecast to reach USD 15.10 billion by 2030, advancing at a 9.11% CAGR.
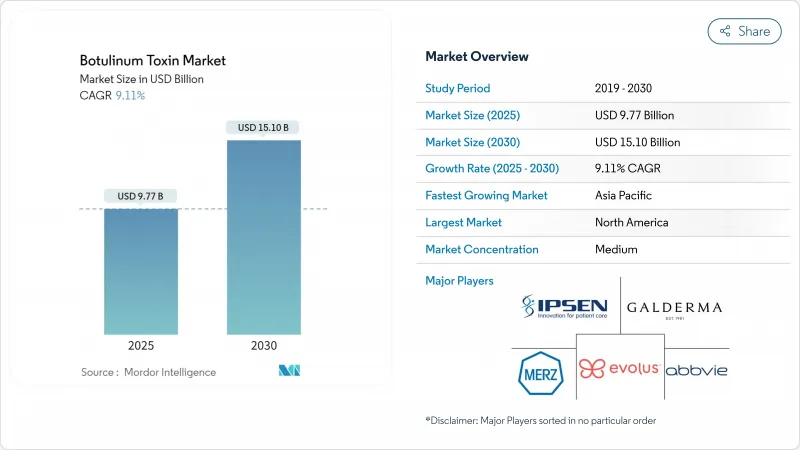
Therapeutic versatility is widening demand beyond cosmetic use, with long-acting serotypes, precision-dosing devices, and new psychiatric indications setting the growth tempo. An expanding male consumer base, rising medical approvals in Asia-Pacific, and technology-enabled dosing accuracy are reinforcing commercial momentum. Strategic acquisitions, such as Crown Laboratories' buyout of Revance Therapeutics for USD 924 million in 2024, are intensifying competition while redefining duration-led product segments. Regulatory vigilance against counterfeit products, coupled with reimbursement shifts in North America and Europe, creates near-term friction yet also spurs investment in secure supply chains and traceable packaging.
Global Botulinum Toxin Market Trends and Insights
Increasing Demand for Aesthetic Procedures
Male uptake is rising as more than 300,000 men opted for injections in 2024, reflecting cultural shifts toward routine grooming. Higher dosing requirements linked to greater muscle mass enlarge per-patient revenue. Preventative treatments in the 25-35 age cohort extend lifetime treatment cycles and heighten long-term sales. Adoption is accelerating in traditionally conservative Middle Eastern markets, indicating quickly eroding cultural barriers. Together, these factors create resilient, demographically diverse demand able to weather macro-economic swings.
Growing R&D for Expanded Therapeutic Uses
Phase III trials show efficacy in depression and anxiety via the facial-feedback mechanism. Cardiac surgery studies demonstrate potential in post-operative atrial fibrillation, hinting at multi-billion-dollar hospital revenue streams. Neurological pipeline programs target Parkinson's disease and trigeminal neuralgia, while pediatric approvals for cerebral palsy broaden recurrence-driven sales. Precision-medicine approaches leveraging genetic markers promise higher efficacy and fewer side effects, reinforcing physician confidence and payer support.
Adverse Effects & Safety Concerns
Regulators are investigating 17 adverse events linked to counterfeit imports across nine U.S. states, heightening practitioner vigilance and driving up compliance costs. FDA crackdowns underscore supply-chain vulnerabilities, prompting wider adoption of tamper-evident packaging and serialization. Neutralizing antibodies, although infrequent, reduce long-term efficacy for chronic therapies, nudging R&D toward serotype diversity. Rare toxin spread events amplify malpractice premiums and may discourage small-practice participation.
Other drivers and restraints analyzed in the detailed report include:
- Precision-Dosing Injection Devices Cut Wastage
- Psychiatric-Disorder Trials Entering Phase II
- Limited Reimbursement of Cosmetic Procedures
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Type A recorded a 91.67% botulinum toxin market share in 2024, reflecting its long safety record and broad indication roster. Type B, though niche, is expanding at 9.83% CAGR, catering to antibody-positive patients and spasticity cases. Extended-duration formulations such as Daxxify deliver median results of 24 weeks, upending the traditional 12-week cycle. Liquid candidates now in trials promise reconstitution-free handling and improved shelf life. Competitive intensity is rising as Korean-origin Letybo gains U.S. approval, challenging Botox's brand equity. The product landscape is moving toward duration- and formulation-based categories that emphasize patient convenience and clinic efficiency.
Type A still anchors most research funding and physician preference, yet payers may gravitate to cost-advantaged biosimilars once patents lapse. Type B suppliers are exploring targeted delivery to autonomic disorders, broadening their clinical narrative. The growing array of serotypes and formulations complicates stocking decisions for providers but offers patients customized options tailored to treatment frequency, onset speed, and antibody profile.
Cosmetic injections retained 62.39% revenue in 2024, but therapeutic procedures are growing faster at 10.66% CAGR as evidence mounts for neurologic, cardiac, and psychiatric benefits. Chronic migraine prevention remains a revenue cornerstone thanks to durable efficacy over multiple 12-week cycles. Depression protocols using glabellar injections yielded 44.8% response rates, underscoring psychiatry's revenue potential. Cardiac surgery trials report reduced postoperative atrial fibrillation episodes, positioning neurotoxins within hospital cardiac suites.
The botulinum toxin market size for therapeutic applications is projected to climb sharply as regulators approve additional indications. Health Canada's higher dosing ceiling for upper-limb spasticity illustrates growing clinical confidence . Insurance coverage is stronger for medical uses, creating predictable billing streams that offset elective-cosmetic cyclicality. Overall, therapeutic expansion is rebalancing sales portfolios toward medically necessary segments with stable reimbursement.
The Botulinum Toxin Market Report Segments the Industry Into by Product (Botulinum Toxin Type A, Botulinum Toxin Type B), by Application (Cosmetic Applications, Therapeutic Applications), by Gender (Male and Female) by End User (Spas and Beauty Centers, Clinics and Hospitals, and More), and Geography. The Market Sizes and Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America preserved 43.27% revenue share in 2024 due to entrenched reimbursement pathways and seasoned injector networks. FDA oversight curtails counterfeit penetration and supports high average selling prices, yet enforcement cases in 2024 revealed persistent gray-market risks. Medicare's reconsideration of therapeutic coverage can unlock incremental growth, even as cosmetic reimbursement stays limited.
Asia-Pacific is the fastest mover, projected at 11.75% CAGR, driven by China's National Medical Products Administration approvals and an expanding middle class. Western manufacturers form distribution alliances with local partners to navigate regional tender systems. Korean innovators, propelled by lower production costs and proactive regulatory engagement, are penetrating Japan, Thailand, and the United States, shifting competitive dynamics.
Europe shows steady but slower expansion, aided by pan-EU clinical trial networks that advance new indications. Harmonized CE certification reduces time-to-market for device-aided delivery systems. Latin America and the Middle East & Africa collectively represent a rising frontier where biosimilar approvals and localized fill-finish plants are boosting access. Industrial diversification of manufacturing sites-to the UAE for Daewoong and to Brazil for Medytox-underscores the drive toward regional self-sufficiency and supply-chain resilience.
- Abbvie
- Ipsen
- Merz Pharma
- Galderma
- Revance Therapeutics
- Evolus
- Medytox
- Daewoong Pharmaceutical
- Hugel
- Huons Global
- Supernus Pharmaceuticals
- Lanzhou Institute of Biological Products
- Chong Kun Dang Pharma
- Alphaeon
- Ipsen Biopharm Ltd.
- Huadong Medicine
- Suneva Medical
- Croma-Pharma
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increasing Demand For Aesthetic Procedures
- 4.2.2 Growing R&D For Expanded Therapeutic Indications
- 4.2.3 Precision-Dosing Injection Devices Cut Wastage
- 4.2.4 Psychiatric-Disorder Indications Entering Phase Ii
- 4.2.5 Commercialization Of Longer-Duration Neurotoxins
- 4.2.6 Biosimilar Type-A Approvals In Emerging Markets
- 4.3 Market Restraints
- 4.3.1 Adverse Effects & Safety Concerns
- 4.3.2 Limited Reimbursement Of Cosmetic Procedures
- 4.3.3 Counterfeit/Illegal Injectables Prompt Crackdowns
- 4.3.4 Neutralizing Antibodies Reduce Long-Term Efficacy
- 4.4 Value / Supply-Chain Analysis
- 4.5 Regulatory Landscape
- 4.6 Technological Outlook
- 4.7 Porter's Five Forces Analysis
- 4.7.1 Threat of New Entrants
- 4.7.2 Bargaining Power of Buyers
- 4.7.3 Bargaining Power of Suppliers
- 4.7.4 Threat of Substitutes
- 4.7.5 Competitive Rivalry
5 Market Size & Growth Forecasts (Value)
- 5.1 By Product
- 5.1.1 Botulinum Toxin Type A
- 5.1.2 Botulinum Toxin Type B
- 5.2 By Application
- 5.2.1 Cosmetic Applications
- 5.2.1.1 Glabellar Lines
- 5.2.1.2 Lateral Canthal Lines (Crow's Feet)
- 5.2.1.3 Forehead Lines
- 5.2.1.4 Masseter Hypertrophy
- 5.2.1.5 Lip Flip & Gummy Smile
- 5.2.2 Therapeutic Applications
- 5.2.2.1 Dystonia
- 5.2.2.2 Chronic Migraine
- 5.2.2.3 Spasticity
- 5.2.2.4 Overactive Bladder
- 5.2.2.5 Sialorrhea
- 5.2.2.6 Ophthalmologic Disorders
- 5.2.2.7 Gastro-oesophageal Disorders (Achalasia)
- 5.2.2.8 Psychiatric Disorders (Depression, Anxiety)
- 5.2.1 Cosmetic Applications
- 5.3 By Gender
- 5.3.1 Male
- 5.3.2 Female
- 5.4 By End User
- 5.4.1 Spas & Beauty Centers
- 5.4.2 Dermatology & Aesthetic Clinics
- 5.4.3 Hospitals & Specialty Centers
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East & Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East & Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 AbbVie
- 6.3.2 Ipsen
- 6.3.3 Merz Pharma
- 6.3.4 Galderma
- 6.3.5 Revance Therapeutics
- 6.3.6 Evolus
- 6.3.7 Medytox
- 6.3.8 Daewoong Pharmaceutical
- 6.3.9 Hugel
- 6.3.10 Huons Global
- 6.3.11 Supernus Pharmaceuticals
- 6.3.12 Lanzhou Institute of Biological Products
- 6.3.13 Chong Kun Dang Pharma
- 6.3.14 Alphaeon
- 6.3.15 Ipsen Biopharm Ltd.
- 6.3.16 Huadong Medicine
- 6.3.17 Suneva Medical
- 6.3.18 Croma-Pharma
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-need Assessment




