PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851260

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851260
Molecular Diagnostics - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The molecular diagnostics market is valued at USD 17.94 billion in 2025 and is forecast to reach USD 28.49 billion by 2030, advancing at a 9.68% CAGR through the period.
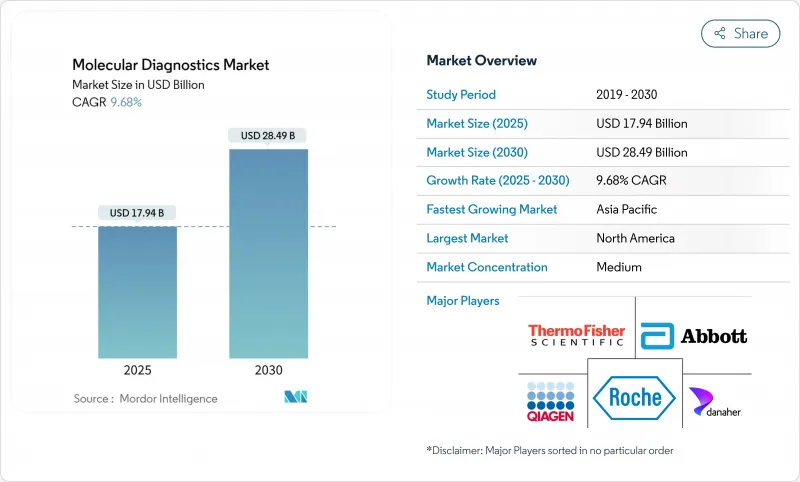
Growth is underpinned by wider adoption of rapid multiplex testing in emergency and outpatient settings, stronger reimbursement for precision oncology assays, and sustained investments in national genome initiatives that improve reference databases. North America keeps a leading position because of established payer frameworks and accelerated retail-pharmacy testing, while Asia Pacific posts the quickest revenue climb on the back of ambitious population-genomics projects and expanding laboratory infrastructure. Technology suppliers are prioritizing integrated platforms that combine PCR, isothermal and sequencing-based workflows, a strategy that shortens turnaround and lowers per-test costs. At the same time, retailers and primary-care networks are capitalizing on streamlined CLIA rules that enable molecular testing at the consumer's first point of contact, shifting volumes away from traditional laboratories.
Global Molecular Diagnostics Market Trends and Insights
Syndromic respiratory panels accelerating point-of-care PCR demand
Multiplex respiratory panels simultaneously detect multiple pathogens and now deliver results in 45-90 minutes, a shift that improves early therapy and isolation decisions in intensive-care units. Clinical adoption has lowered unnecessary antibiotic prescriptions by 20-30%, reinforcing stewardship initiatives. Because these assays also uncover co-infections in roughly 20% of respiratory cases, they reduce diagnostic blind spots that are common with single-pathogen tests. Hospital networks are codifying panel use within infection-control protocols, ensuring year-round volumes instead of seasonal peaks. As a result, suppliers of cartridge-based systems report sustained reagent demand, a trend that lifts recurring revenue and underlines the molecular diagnostics market's focus on decentralization.
CLIA-waived molecular devices entering retail pharmacies
Revisions to U.S. CLIA rules effective December 2024 simplified personnel requirements and fee structures, enabling pharmacies to run moderate-complexity molecular assays. CVS Health introduced a three-in-one combo PCR for influenza A/B and COVID-19 across 1,600 outlets, giving customers testing and prescription consultation in a single visit. Kroger followed with cholesterol and glucose molecular testing across 2,100 sites, shrinking screening time to 90 seconds. The move repositions pharmacies as first-line diagnostic hubs, broadening access for underserved communities and redirecting sample flow away from central laboratories. Manufacturers of portable platforms benefit from higher test frequency and consumer visibility, supporting long-term revenue streams in the molecular diagnostics market.
Enzyme supply chain constraints causing cost spikes
Medical device makers have watched logistics and raw-material expenses climb to nearly 20% of revenue as geopolitical tensions disrupt trade lanes. High-purity enzymes are particularly exposed, with PTFE shortages forcing contract manufacturers to insource capabilities. Smaller assay developers struggle to secure buffer components and stabilizers, prompting production delays, higher list prices and slower entry into new markets. In emerging economies, where price sensitivity is acute, the resulting unit-cost escalation inhibits test adoption, capping near-term expansion of the molecular diagnostics market.
Other drivers and restraints analyzed in the detailed report include:
- EMA-approved companion diagnostics boosting oncology test volumes
- Population genomics initiatives driving NGS test uptake
- EU IVDR backlog delaying new assay commercialization
For complete list of drivers and restraints, kindly check the Table Of Contents.
List of Companies Covered in this Report:
- Roche
- Abbott Laboratories
- Thermo Fisher Scientific
- Danaher
- Hologic
- Illumina
- QIAGEN
- Beckton Dickinson
- bioMerieux
- Agilent Technologies
- Bio-Rad Laboratories
- Sysmex
- Siemens Healthineers
- DiaSorin
- Seegene
- Guardant Health
- LabCorp
- Exact Sciences
- 10x Genomics
- DNA Genotek, Inc.
- PathoNostics B.V.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Syndromic Respiratory Panels Adoption Accelerating PoC PCR Demand in North America
- 4.2.2 CLIA-Waived Molecular Devices Entering Retail Pharmacies Expanding Access
- 4.2.3 EMA-Approved Companion Diagnostics Boosting Oncology Test Volumes in Europe
- 4.2.4 Population Genomics Initiatives Driving NGS Test Uptake Across APAC
- 4.2.5 AI-Enabled Bioinformatics Pipelines Shortening Result Turnaround in High-Throughput Labs
- 4.2.6 National Antimicrobial-Resistance Surveillance Programs Fueling Multiplex PCR Panel Procurement in Hospitals
- 4.3 Market Restraints
- 4.3.1 Enzyme Supply Chain Constraints Causing Cost Spikes for PCR Kits
- 4.3.2 EU IVDR Backlog Delaying New Assay Commercialization
- 4.3.3 Limited Reimbursement Coverage for Comprehensive NGS Panels
- 4.3.4 Stringent Data-Privacy Regulations Hindering Cloud-Based Result Delivery
- 4.4 Regulatory or Technological Outlook
- 4.5 Porter's Five Forces Analysis
- 4.5.1 Bargaining Power of Buyers
- 4.5.2 Bargaining Power of Suppliers
- 4.5.3 Threat of New Entrants
- 4.5.4 Threat of Substitute Products
- 4.5.5 Intensity of Competitive Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Technology
- 5.1.1 PCR
- 5.1.2 Next-Generation Sequencing (NGS)
- 5.1.3 In Situ Hybridization
- 5.1.4 Chips & Microarrays
- 5.1.5 Mass Spectrometry
- 5.1.6 Other Technologies
- 5.2 By Application
- 5.2.1 Infectious Disease
- 5.2.2 Oncology
- 5.2.3 Pharmacogenomics
- 5.2.4 Microbiology
- 5.2.5 Genetic Disease Screening
- 5.2.6 Human Leukocyte Antigen Typing
- 5.2.7 Blood Screening
- 5.3 By Product
- 5.3.1 Reagents & Kits
- 5.3.2 Instruments & Systems
- 5.3.3 Software & Services
- 5.4 By Sample Type
- 5.4.1 Blood, Serum & Plasma
- 5.4.2 Urine
- 5.4.3 Other Sample Types (Saliva, Tissue, Swabs)
- 5.5 By End User
- 5.5.1 Hospitals
- 5.5.2 Diagnostic & Reference Laboratories
- 5.5.3 Academic & Research Institutes
- 5.5.4 Other End Users
- 5.6 By Geography
- 5.6.1 North America
- 5.6.1.1 United States
- 5.6.1.2 Canada
- 5.6.1.3 Mexico
- 5.6.2 Europe
- 5.6.2.1 Germany
- 5.6.2.2 United Kingdom
- 5.6.2.3 France
- 5.6.2.4 Italy
- 5.6.2.5 Spain
- 5.6.2.6 Rest of Europe
- 5.6.3 Asia-Pacific
- 5.6.3.1 China
- 5.6.3.2 Japan
- 5.6.3.3 India
- 5.6.3.4 Australia
- 5.6.3.5 South Korea
- 5.6.3.6 Rest of Asia-Pacific
- 5.6.4 Middle East & Africa
- 5.6.4.1 GCC
- 5.6.4.2 South Africa
- 5.6.4.3 Rest of Middle East & Africa
- 5.6.5 South America
- 5.6.5.1 Brazil
- 5.6.5.2 Argentina
- 5.6.5.3 Rest of South America
- 5.6.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Strategic Moves
- 6.3 Market Share Analysis
- 6.4 Company Profiles (includes Global level Overview, Market level overview, Core Business Segments, Financials, Headcount, Key Information, Market Rank, Market Share, Products and Services, and analysis of Recent Developments)
- 6.4.1 F. Hoffmann-La Roche Ltd
- 6.4.2 Abbott Laboratories
- 6.4.3 Thermo Fisher Scientific Inc.
- 6.4.4 Danaher
- 6.4.5 Hologic Inc.
- 6.4.6 Illumina Inc.
- 6.4.7 Qiagen N.V.
- 6.4.8 Becton, Dickinson and Company
- 6.4.9 bioMrieux SA
- 6.4.10 Agilent Technologies Inc.
- 6.4.11 Bio-Rad Laboratories Inc.
- 6.4.12 Sysmex Corporation
- 6.4.13 Siemens Healthineers AG
- 6.4.14 DiaSorin S.p.A.
- 6.4.15 Seegene Inc.
- 6.4.16 Guardant Health
- 6.4.17 Labcorp
- 6.4.18 Exact Sciences Corporation
- 6.4.19 10x Genomics
- 6.4.20 DNA Genotek, Inc.
- 6.4.21 PathoNostics B.V.
7 Market Opportunities & Future Outlook
- 7.1 White-Space & Unmet-Need Assessment




