PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1432797

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1432797
Global Pregnancy Detection Kits - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2024 - 2029)
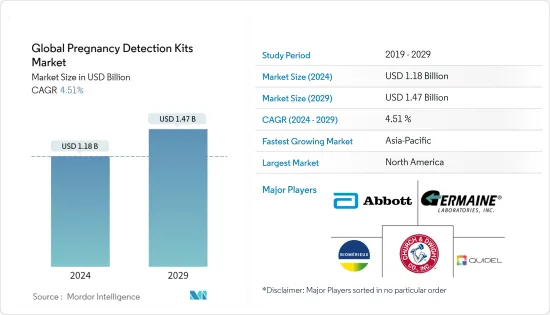
The Global Pregnancy Detection Kits Market size is estimated at USD 1.18 billion in 2024, and is expected to reach USD 1.47 billion by 2029, growing at a CAGR of 4.51% during the forecast period (2024-2029).
The outbreak of the COVID-19 pandemic has pushed the pharmaceutical industry into action, with a race to develop both therapeutic and preventive drugs. Exponentially increasing cases of the coronavirus worldwide are leading to the need for the development of novel treatments, with several clinical trials underway. The COVID-19 pandemic significantly impacted the market. For instance, according to a research study by Chiu-Lin Wang et al., published in the International Journal of Medical Sciences in January 2021, pregnant women may be at a higher risk of being infected with COVID-19 and may develop more complicated clinical events. Thus, as a result, the sale of pregnancy detection kits has increased during the pandemic. However, the majority of pregnant women who were infected with COVID-19 were found to be asymptomatic. For instance, according to the Royal College of Obstetricians and Gynaecologists' 2021 report, about 74.0% of pregnant women were asymptomatic compared to non-pregnant women with COVID-19, pregnant women with COVID-19 have higher rates of intensive care unit (ICU) admission. Therefore, the market witnessed moderate growth during the pandemic.
The factors that are driving the market growth include an increase in unplanned pregnancies, a rise in disposable income along with changing lifestyles, and growing literacy rates among women. Uttar Pradesh, the most populous state in India has twice the unwanted fertility rate as that seen for India as a whole. For instance, according to a research study by Nabamallika Dehingia et al., published in BMC Pregnancy and Childbirth Journal, in March 2020, around one-fifth of the women (16.9%) in Uttar Pradesh, India, reported that their previous pregnancy was unintended, and 44.0% pregnancies in Europe and 38.0% in Asia are unplanned, leading to high demand for pregnancy detection kits. In addition, according to a research study by Jonathan Bearak et al., published in Lancet Journal, in September 2020, during the period 2015-2019, globally, there were around 121.0 million unintended pregnancies annually, corresponding to a global rate of 64 unintended pregnancies per 1,000 women who are aged 15-49 years. The high rate of unplanned pregnancies is boosting the market growth. Adding to that, a pregnancy detection kit is one of the primary and easy ways for women to check if they are pregnant or not. Thus, the demand for pregnancy detection kits is expected to increase in the future.
However, women in low-income countries are not properly aware of pregnancy detection kits, and most of the time, such kits are not easily accessible to such women. Easy-to-use, highly accurate dip-strip tests can be purchased from manufacturers for less than ten cents. Furthermore, in low-income countries, pregnancy test kits are not readily available and the lack of awareness along with a low literacy rate of women further restricts the sale of pregnancy kits in these countries. Thus, factors, such as lack of awareness and lack of availability of pregnancy test kits, are likely to impede the growth of the market studied.
Pregnancy Detection Kits Market Trends
Blood test for HCG Segment is Expected to Witness Growth Over the Forecast Period
The human chorionic gonadotropin (HCG) blood test is a quantitative test that measures the level of hCG hormone present in a sample of the blood. hCG is a hormone produced in the female body during pregnancy.
COVID-19 is a current global public health emergency. COVID-19 has left an indelible mark on several domains, and the healthcare industry is no exception. According to a research study by Stefano Cosma et al., published in the American Journal of Obstetrics and Gynecology, in April 2021, severe acute respiratory syndrome coronavirus 2 infections during the first trimester of pregnancy do not seem to predispose to early pregnancy loss, and its cumulative incidence did not differ between women with spontaneous abortion and women with ongoing pregnancy. Moreover, COVID-19 appears to have a favorable maternal course at the beginning of pregnancy, consistent with what has been observed during the second and third trimesters. According to a research study by Matan Anteby et al., published in the Lancet Journal, in January 2021, during the COVID-19 pandemic 2020, there was a 28.3% reduction in women seeking early pregnancy and emergent gynecological medical care. In addition, Prestige Brands, 2020 has also stated that 75.0% of women prefer self-testing for pregnancy over laboratory tests. This ascertains the growth of self-testing kits during COVID-19.
Unintended pregnancy is a significant public health concern in South Asian countries. For instance, according to a research study by Alamgir Sarder et al., published in PLOS ONE Journal, in February 2021, around 19.1% of pregnancies were reported as unintended ranging from 11.9% in India to 28.4% in Bangladesh. Moreover, younger women who are aged 15-19 years had a 1.42 times higher chance of unintended pregnancies. During pregnancy, the cells in the developing placenta produce hCG. The placenta in the sacs nourishes the egg after it is fertilized and attaches to the uterine wall. The hCG hormone is first detected in the female blood sample about 11 days after conception, and the level of hCG continues to double after 48 to 72 hours. Once they reach the peak- which occurs around 8 to 11 weeks after conception, the hCG levels decline and then remain stable for the rest of the pregnancy. This HCG test is performed to confirm the pregnancy, determine the age of the fetus, diagnose a potential miscarriage, and diagnose an abnormal pregnancy. Therefore, due to the growth of planned and unplanned pregnancies, blood HCG tests are recommended by doctors, which helps to drive the segment market.
Therefore, due to the above-mentioned factors, the blood test for the HCG segment is anticipated to witness considerable growth over the forecast period.
North America is Expected to Dominate the Pregnancy Detection Kits Market
Some of the factors that are driving the market growth in the North American region include a growing increase in unplanned pregnancies, awareness regarding pregnancy detection kits, growing expenditure on personal care products, and the presence of key market players.
According to the Centers for Disease Control and Prevention (CDC) report, May 2021, pregnant women with COVID-19 are at increased risk for preterm birth and might have an increased risk of other adverse pregnancy outcomes. In the North American region, the pregnancy detection kits market is expected to witness an upswing as the demand for self-test kits and point-of-care kits will increase due to people preferring to stay at home instead of visiting hospitals during the ongoing pandemic. For instance, according to the statistics from Statistics Canada 2020, the sales of family planning products such as pregnancy tests, and contraceptives have been way up during the coronavirus pandemic in Canada.
In Canada, unintended pregnancies in adolescents significantly impact young women, their families/children, and society. For instance, according to a research study by Amanda Black et al., published in the Journal of Obstetrics and Gynaecology Canada, in May 2019, among Canadian adolescents, there were more than 39,000 unintended pregnancies (UPs) annually. The market players are adopting various strategies such as product launches, developments, collaborations, acquisitions, and expansions to increase market share. For instance, in May 2019, True Diagnostics, Inc., received 510(k) marketing clearance from the United States Food and Drug Administration (FDA) for the VeriClear Digital Early Result Pregnancy Test for detecting human chorionic gonadotropin (hCG) levels in women before their first missed period. Thus, owing to the above factors, the market is expected to witness significant growth over the forecast period.
Pregnancy Detection Kits Industry Overview
The pregnancy detection kits market is moderately competitive and consists of a number of players. However, with technological advancements and product innovation, mid-size to smaller companies are increasing their market presence by introducing new products with cost-effective pricing. Some of the market players include Abbott Laboratories, BioMerieux SA, Church & Dwight Co, Inc., Quidel Corporation, Germaine Laboratories Inc, Cardinal Health Inc, Axis Medicare, Mankind Pharma, Piramal Enterprises, and Procter & Gamble Co.
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 INTRODUCTION
- 1.1 Study Assumptions and Market Definition
- 1.2 Scope of the Study
2 RESEARCH METHODOLOGY
3 EXECUTIVE SUMMARY
4 MARKET DYNAMICS
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Increase in Unplanned Pregnancies
- 4.2.2 Rise in Disposable Income
- 4.2.3 Changing Lifestyles and Growing Women Literacy Rate
- 4.3 Market Restraints
- 4.3.1 Lack of Availability and Awareness in Low Income Countries
- 4.4 Porter's Five Force Analysis
- 4.4.1 Threat of New Entrants
- 4.4.2 Bargaining Power of Buyers/Consumers
- 4.4.3 Bargaining Power of Suppliers
- 4.4.4 Threat of Substitute Products
- 4.4.5 Intensity of Competitive Rivalry
5 MARKET SEGMENTATION (Market Size by Value - USD Million)
- 5.1 By Product Type
- 5.1.1 Line Pregnancy Tests
- 5.1.1.1 Test Cassette Format
- 5.1.1.2 Test Strip Format
- 5.1.1.3 Test Midstream Format
- 5.1.2 Digital Devices
- 5.1.1 Line Pregnancy Tests
- 5.2 By Type of Test
- 5.2.1 Urine test for HCG
- 5.2.2 Blood test for HCG
- 5.3 By Distribution Channel
- 5.3.1 Hospital Pharmacies
- 5.3.2 Independent Pharmacies and Drug Store
- 5.3.3 Online Pharmacies
- 5.3.4 Other Distribution Channels
- 5.4 Geography
- 5.4.1 North America
- 5.4.1.1 United States
- 5.4.1.2 Canada
- 5.4.1.3 Mexico
- 5.4.2 Europe
- 5.4.2.1 Germany
- 5.4.2.2 United Kingdom
- 5.4.2.3 France
- 5.4.2.4 Italy
- 5.4.2.5 Spain
- 5.4.2.6 Rest of Europe
- 5.4.3 Asia-Pacific
- 5.4.3.1 China
- 5.4.3.2 Japan
- 5.4.3.3 India
- 5.4.3.4 Australia
- 5.4.3.5 South Korea
- 5.4.3.6 Rest of Asia-Pacific
- 5.4.4 Middle East and Africa
- 5.4.4.1 GCC
- 5.4.4.2 South Africa
- 5.4.4.3 Rest of Middle East and Africa
- 5.4.5 South America
- 5.4.5.1 Brazil
- 5.4.5.2 Argentina
- 5.4.5.3 Rest of South America
- 5.4.1 North America
6 COMPETITIVE LANDSCAPE
- 6.1 Company Profiles
- 6.1.1 Abbott Laboratories
- 6.1.2 Axis Medicare
- 6.1.3 BioMerieux SA
- 6.1.4 Cardinal Health Inc.
- 6.1.5 Church & Dwight Co, Inc.
- 6.1.6 Germaine Laboratories Inc.
- 6.1.7 Mankind Pharma
- 6.1.8 Piramal Enterprises Ltd
- 6.1.9 Procter & Gamble Co.
- 6.1.10 Quidel Corporation
- 6.1.11 SPD Swiss Precision Diagnostics
7 MARKET OPPORTUNITIES AND FUTURE TRENDS




