PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851901

PUBLISHER: Mordor Intelligence | PRODUCT CODE: 1851901
Drug Device Combination Products - Market Share Analysis, Industry Trends & Statistics, Growth Forecasts (2025 - 2030)
The drug device combination products market stood at USD 140.7 billion in 2025 and is forecast to reach USD 186.52 billion by 2030, advancing at a 5.8% CAGR fda.gov.
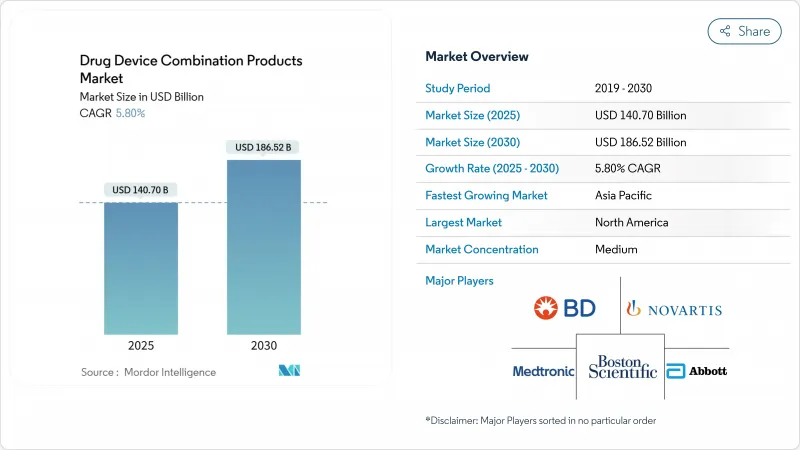
Growth stems from rising chronic-disease prevalence, faster U.S. regulatory pathways that trim approval timelines , and patient demand for integrated therapies that improve adherence while lowering overall care costs. Convergence of real-time monitoring with targeted drug delivery is turning once-passive devices into active disease-management platforms, creating fresh value propositions for payers and providers. North America keeps its lead through robust innovation funding, whereas Asia-Pacific gains momentum on cost-competitive sterile manufacturing and supportive policy harmonization. Competitive activity intensifies as incumbents buy niche innovators to secure drug-device know-how and digital capabilities.
Global Drug Device Combination Products Market Trends and Insights
Growing Burden of Chronic Illnesses Driving Demand for Targeted Combination Therapies
Cardiovascular disease affects 655 million people worldwide, sustaining demand for drug-eluting stents and drug-coated balloons that unite mechanical support with localized pharmacotherapy. The FDA-cleared AGENT paclitaxel-coated balloon cut major adverse cardiac events by 11.1% versus uncoated devices, reinforcing the clinical edge of integrated platforms . Diabetes solutions that couple continuous glucose monitoring with automated insulin dosing now achieve 70% glycemic-control rates, far higher than traditional regimens. Oncology is moving toward intra-operative imaging-drug hybrids such as Lumisight, which delivers 84% diagnostic accuracy and limits repeat surgeries. These examples show how drug device combination products market innovators lower long-term costs by pairing precision diagnostics with therapy .
Rapidly Ageing Population Boosting Uptake of Self-Administered Delivery Formats
The global population aged 65+ will reach 771 million by 2030, amplifying need for easy-to-use devices that offset declining dexterity. Autoinjectors like Ypsomed's YpsoDose use audio-visual cues to aid seniors. Home care settings-already the fastest-growing end-user at a 6.56% CAGR-benefit from patch pumps such as Embecta's smartphone-enabled insulin system that lets patients treat themselves safely. Real-time adherence data address non-compliance, which affects half of chronic-disease patients. The FDA's human-factors guidance steers manufacturers toward designs that older adults can use correctly on first attempt.
Multicentre Regulatory Compliance Adds Cost & Delays
Europe's MDR and IVDR expansions raise documentation burdens, adding 8-12 months to approvals versus earlier rules. Notified-body reviews elevate compliance costs 25-40% for combination developers. Divergent FDA-EMA classifications force duplicate studies, while Asia-Pacific remains fragmented despite ASEAN harmonization efforts. Without mutual recognition, firms must maintain separate quality systems, inflating overhead and slowing global roll-out of novel therapies within the drug device combination products market.
Other drivers and restraints analyzed in the detailed report include:
- Breakthroughs in Minimally-Invasive & Smart Delivery Platforms
- US FDA's Expedited Combo-Product Pathways Shortening Time-to-Market
- High Recall Rates Linked to Sterility / Dose Accuracy
For complete list of drivers and restraints, kindly check the Table Of Contents.
Segment Analysis
Drug-eluting stents retained leadership with a 24.56% share in 2024, reflecting decades of evidence for reduced restenosis. Continued polymer advances extend drug elution beyond 180 days, preserving the segment's dominance in the drug device combination products market. Prefilled syringes, however, are charting a 6.34% CAGR through 2030 as biologics volumes climb and smart sensors log injection events for payers. The drug device combination products market size for prefilled systems is projected to climb markedly as connectivity standards mature and value-based care rewards adherence tracking.
Transdermal patches now use microneedles for macromolecules, expanding into weight-loss therapies. Autoinjectors grow on ergonomic refinements, while wearable injectors turn basal-insulin delivery into a discreet patch experience. Connected inhalers that pair with mobile apps improved asthma-control scores 43% versus conventional devices. Infusion pumps miniaturize for hepatic-artery cancer infusions following Boston Scientific's Intera Oncology buy, strengthening its presence inside the drug device combination products market.
Cardiovascular therapies claimed 35.51% of 2024 revenue, anchored by drug-eluting stents, coated balloons, and rhythm-management implants. Pulsed-field ablation represents the next cardiac frontier and keeps the segment a mainstay in the drug device combination products market. Pain management, however, is on a 6.45% CAGR path as implantable neuromodulation offers a non-opioid alternative and garners insurance coverage. The drug device combination products market size for neuromodulation systems is projected to rise quickly as chronic-pain prevalence climbs.
Diabetes maintains robust momentum through sensor-pump ecosystems. Respiratory disorders benefit from digital inhalers and novel formulations for pulmonary hypertension. Oncology is shifting toward imaging-guided drug delivery that provides immediate feedback on therapeutic placement. Obesity implants and mental-health patches represent emerging categories that could further diversify the drug device combination products industry.
The Drug Device Combination Products Market Report is Segmented by Product (Drug-Eluting Stents, Transdermal Patches, Infusion Pumps, and More), Application (Cardiovascular Diseases, Diabetes, Cancer Therapy, and More), End-User (Hospitals and Clinics, and More), Route of Administration (Oral, Parenteral, and More), and Geography (North America, Europe, and More). The Market Forecasts are Provided in Terms of Value (USD).
Geography Analysis
North America contributed 40.56% of global revenue in 2024, supported by the FDA's clear regulatory architecture and high healthcare spending. Large acquisitions, such as Johnson & Johnson's USD 12.5 billion Shockwave Medical buy, augment cardiac-intervention portfolios and reinforce regional dominance. Digital-health partnerships flourish, enabling connected glucose-monitoring ecosystems that further entrench the drug device combination products market.
Asia-Pacific delivers the fastest 6.82% CAGR through 2030. Manufacturing clusters in China, India, and Vietnam slash sterile-assembly costs, while initiatives like the ASEAN Medical Device Directive streamline cross-border registration. Governments fund R&D tax incentives, and regional CDMOs win global outsourcing contracts, broadening market reach. Singapore and South Korea attract clinical-trial activity through efficient ethics-approval cycles.
Europe continues moderate progress despite MDR-related paperwork. Innovation hubs in Germany, Switzerland, and Ireland lead in connected inhalers and digital neuromodulation. Public-private alliances support pilot programs that integrate reimbursement for data-enabled devices, keeping the region integral to the drug device combination products industry.
- Abbott Laboratories
- Medtronic
- Boston Scientific
- Johnson & Johnson
- Novartis
- GlaxoSmithKline
- Beckton Dickinson
- Terumo
- Stryker
- W. L. Gore & Associates
- Abbvie
- Viatris
- Cook Group
- Hisamitsu Pharmaceutical
- Teva Pharmaceutical Industries
- Solventum
- Phillips-Medisize
- Ypsomed
- AstraZeneca
- Novo Nordisk
Additional Benefits:
- The market estimate (ME) sheet in Excel format
- 3 months of analyst support
TABLE OF CONTENTS
1 Introduction
- 1.1 Study Assumptions & Market Definition
- 1.2 Scope of the Study
2 Research Methodology
3 Executive Summary
4 Market Landscape
- 4.1 Market Overview
- 4.2 Market Drivers
- 4.2.1 Growing Burden Of Chronic Illnesses Driving Demand for Targeted Combination Therapies
- 4.2.2 Rapidly Ageing Population Boosting Uptake of Self-Administered Delivery Formats
- 4.2.3 Breakthroughs In Minimally-Invasive & Smart Delivery Platforms
- 4.2.4 US FDA's Expedited Combo-Product Pathways Shortening Time-To-Market
- 4.2.5 Connected Inhalers & Patches Enabling Payer-Mandated Adherence Analytics
- 4.2.6 Low-Cost Sterile Assembly Capacity Scaling Up Across Asia Lowers ASPs.
- 4.3 Market Restraints
- 4.3.1 Multicentre Regulatory Compliance Adds Cost & Delays
- 4.3.2 High Recall Rates Linked to Sterility / Dose Accuracy
- 4.3.3 Tight Supply of Specialty Polymers Compatible with Apis
- 4.3.4 Absence Of Unified Reimbursement Codes for Digital Combo Devices
- 4.4 Regulatory Landscape
- 4.5 Technological Outlook
- 4.6 Porters Five Forces Analysis
- 4.6.1 Threat of New Entrants
- 4.6.2 Bargaining Power of Buyers
- 4.6.3 Bargaining Power of Suppliers
- 4.6.4 Threat of Substitutes
- 4.6.5 Intensity of Rivalry
5 Market Size & Growth Forecasts (Value, USD)
- 5.1 By Product
- 5.1.1 Drug-Eluting Stents
- 5.1.2 Transdermal Patches
- 5.1.3 Infusion Pumps
- 5.1.4 Drug-Coated Balloons
- 5.1.5 Inhalers
- 5.1.6 Prefilled Syringes
- 5.1.7 Wearable Injectors
- 5.1.8 Autoinjectors
- 5.1.9 Others
- 5.2 By Application
- 5.2.1 Cardiovascular Diseases
- 5.2.2 Diabetes
- 5.2.3 Cancer Therapy
- 5.2.4 Respiratory Disorders
- 5.2.5 Pain Management
- 5.2.6 Others
- 5.3 By End-User
- 5.3.1 Hospitals and Clinics
- 5.3.2 Ambulatory Surgical Centers
- 5.3.3 Home Care Settings
- 5.3.4 Others
- 5.4 By Route of Administration
- 5.4.1 Oral
- 5.4.2 Parenteral
- 5.4.3 Transdermal
- 5.4.4 Implantable
- 5.4.5 Others
- 5.5 By Geography
- 5.5.1 North America
- 5.5.1.1 United States
- 5.5.1.2 Canada
- 5.5.1.3 Mexico
- 5.5.2 Europe
- 5.5.2.1 Germany
- 5.5.2.2 United Kingdom
- 5.5.2.3 France
- 5.5.2.4 Italy
- 5.5.2.5 Spain
- 5.5.2.6 Rest of Europe
- 5.5.3 Asia-Pacific
- 5.5.3.1 China
- 5.5.3.2 Japan
- 5.5.3.3 India
- 5.5.3.4 Australia
- 5.5.3.5 South Korea
- 5.5.3.6 Rest of Asia-Pacific
- 5.5.4 Middle East and Africa
- 5.5.4.1 GCC
- 5.5.4.2 South Africa
- 5.5.4.3 Rest of Middle East and Africa
- 5.5.5 South America
- 5.5.5.1 Brazil
- 5.5.5.2 Argentina
- 5.5.5.3 Rest of South America
- 5.5.1 North America
6 Competitive Landscape
- 6.1 Market Concentration
- 6.2 Market Share Analysis
- 6.3 Company Profiles (includes Global level Overview, Market level overview, Core Segments, Financials as available, Strategic Information, Market Rank/Share for key companies, Products & Services, and Recent Developments)
- 6.3.1 Abbott Laboratories
- 6.3.2 Medtronic plc
- 6.3.3 Boston Scientific Corporation
- 6.3.4 Johnson & Johnson
- 6.3.5 Novartis AG
- 6.3.6 GlaxoSmithKline plc
- 6.3.7 Becton, Dickinson and Company
- 6.3.8 Terumo Corporation
- 6.3.9 Stryker Corporation
- 6.3.10 W. L. Gore & Associates, Inc.
- 6.3.11 AbbVie
- 6.3.12 Viatris Inc. (Mylan)
- 6.3.13 Cook Medical
- 6.3.14 Hisamitsu Pharmaceutical
- 6.3.15 Teva Pharmaceutical Industries
- 6.3.16 Solventum
- 6.3.17 Phillips-Medisize
- 6.3.18 Ypsomed AG
- 6.3.19 AstraZeneca plc
- 6.3.20 Novo Nordisk
7 Market Opportunities & Future Outlook
- 7.1 White-space & Unmet-Need Assessment




