PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1801873

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1801873
U.S. ATP Assay Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
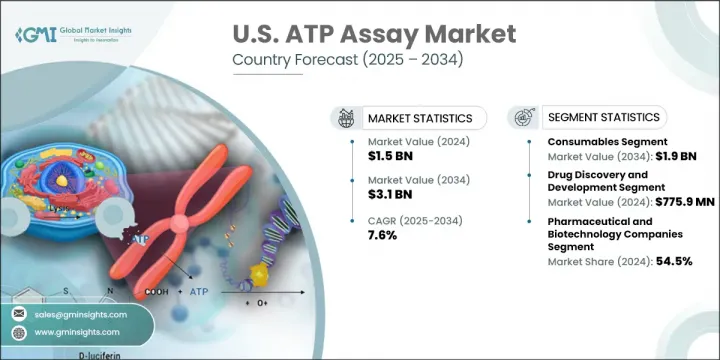
U.S. ATP Assay Market was valued at USD 1.5 billion in 2024 and is estimated to grow at a CAGR of 7.6% to reach USD 3.1 billion by 2034. Growing demand for real-time cellular analysis tools is playing a major role in fueling the market. As the healthcare sector increasingly adopts precision medicine and personalized treatments, ATP assays are becoming essential across diagnostics, research, and pharmaceutical development. These assays offer accurate, real-time data on cell viability and energy metabolism, making them crucial for early-stage drug screening and cytotoxicity studies. The rise in high-throughput screening programs, especially in the biotech and pharma sectors, is further accelerating adoption.
Moreover, regulatory-driven expansion of environmental monitoring practices and contamination control, particularly in water and food safety, is expanding ATP assay applications. As cell-based drug development continues to grow, demand for fast, scalable, and reproducible testing tools is rising. Researchers across clinical and academic institutions rely on ATP-based methods to assess metabolic activity and cell health, thereby enhancing translational research and drug discovery workflows throughout the U.S.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $1.5 Billion |
| Forecast Value | $3.1 Billion |
| CAGR | 7.6% |
The consumables segment dominated the U.S. ATP assay market in 2024, driven by its continuous and indispensable role in laboratory routines. High consumption of assay kits, detection reagents, and microplates is largely attributed to ongoing pharmaceutical R&D, microbial testing, and quality assurance procedures. Their vital use in cell-based viability studies and contamination control ensures regular replenishment, maintaining strong demand across all stages of the research and production process.
The cell-based ATP assays segment will grow at 8% CAGR through 2034. Their rising popularity stems from the ability to deliver consistent, quantitative results with minimal sample prep-ideal for large-scale workflows across biotech, academic, and clinical laboratories. These assays support a broad range of detection systems, offering seamless integration into luminometers, spectrophotometers, and automated analysis tools. Their compatibility and speed make them highly effective in assessing metabolic activity, cell growth, and toxic effects-key factors in the success of drug development and personalized medicine research.
The pharmaceutical and biotech companies segment held 54.5% share in 2024. Their leadership position is backed by strong investment in cell-based therapy development, a deep research infrastructure, and a well-established innovation ecosystem. As industry emphasis shifts toward biologics, gene therapies, and customized treatments, these companies continue to drive high-volume adoption of ATP assays for screening, validation, and safety evaluation purposes.
Leading players shaping the U.S. ATP Assay Market include Promega, Thermo Fisher Scientific, Biotium, Danaher, Cayman Chemical, Merck, Revvity, Cell Signaling Technology, Charm Sciences, Lonza, Berthold Technologies, Agilent Technologies, Abcam, and 3M Company. Companies in the U.S. ATP assay market are enhancing their market presence through a combination of innovation, product portfolio expansion, and strategic collaborations. Many are focusing on launching next-generation assay kits that offer higher sensitivity, speed, and automation compatibility for high-throughput workflows. Firms are also investing in digital integration, enabling real-time data analysis and cloud-based reporting for research labs. Co-development agreements with pharmaceutical companies help tailor assays for specific therapeutic applications. Additionally, players are strengthening their distribution channels and offering bundled solutions that include reagents, instruments, and software.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Product type trends
- 2.2.2 Assay type trends
- 2.2.3 Application trends
- 2.2.4 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.1.1 Supplier landscape
- 3.1.2 Factor affecting the value chain
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Increasing focus on precision medicine and personalized therapies
- 3.2.1.2 Expansion of academic and translational research funding
- 3.2.1.3 Rising burden of chronic diseases
- 3.2.1.4 Growing demand for rapid microbial contamination testing
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of advanced kits
- 3.2.2.2 Limited accessibility in community and rural research settings
- 3.2.3 Market opportunities
- 3.2.3.1 Growing adoption of on-site testing kits for emergency surveillance
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological advancements
- 3.5.1 Current technological trends
- 3.5.2 Emerging technologies
- 3.6 Supply chain analysis
- 3.7 Pricing analysis, 2024
- 3.8 Pipeline and R&D investment analysis
- 3.9 Future market trends
- 3.10 Gap analysis
- 3.11 Porter's analysis
- 3.12 PESTEL analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company market share analysis
- 4.3 Company matrix analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product Type, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Instruments
- 5.2.1 Luminometers
- 5.2.2 Spectrophotometers
- 5.3 Consumables
- 5.3.1 Reagents and kits
- 5.3.2 Microplates
- 5.3.3 Other consumables
Chapter 6 Market Estimates and Forecast, By Assay Type, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 Luminometric ATP assays
- 6.3 Enzymatic ATP assays
- 6.4 Bioluminescence resonance energy transfer (BRET) ATP assays
- 6.5 Cell-based ATP assays
- 6.6 Other assay types
Chapter 7 Market Estimates and Forecast, By Application, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Drug discovery and development
- 7.3 Contamination testing
- 7.4 Disease detection
Chapter 8 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 Pharmaceutical and biotechnology companies
- 8.3 Hospital and diagnostic laboratories
- 8.4 Academic and research institutes
Chapter 9 Company Profiles
- 9.1 3M Company
- 9.2 Abcam
- 9.3 Agilent Technologies
- 9.4 Berthold Technologies
- 9.5 Biotium
- 9.6 Cayman Chemical
- 9.7 Cell Signaling Technology
- 9.8 Charm Sciences
- 9.9 Danaher
- 9.10 Lonza
- 9.11 Merck
- 9.12 Promega
- 9.13 Revvity
- 9.14 Thermo Fisher Scientific




