PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1833641

PUBLISHER: Global Market Insights Inc. | PRODUCT CODE: 1833641
Intrauterine Devices Market Opportunity, Growth Drivers, Industry Trend Analysis, and Forecast 2025 - 2034
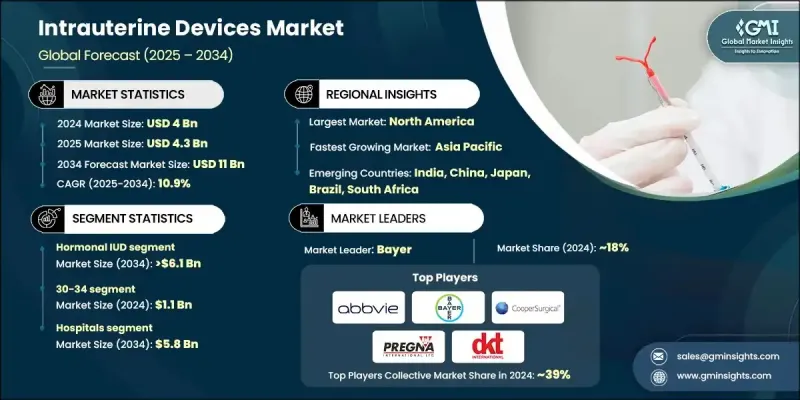
The Global Intrauterine Devices Market was valued at USD 4 billion in 2024 and is estimated to grow at a CAGR of 10.9% to reach USD 11 billion by 2034.
The strong growth trajectory is driven by supportive regulatory policies, greater awareness among women about the diverse uses of IUDs, and the high incidence of unintended pregnancies. IUDs are increasingly acknowledged not only for their role in birth control but also for wider health applications, including the management of heavy menstrual bleeding, endometriosis, and perimenopausal symptoms. With the rise of educational initiatives, digital health platforms, and improved access to reproductive care information, women are now empowered to make more informed decisions about long-term contraceptive options. Healthcare professionals play a significant role by counseling patients on the safety, effectiveness, and additional health benefits of IUDs, which has boosted acceptance globally. Intrauterine devices are small, flexible, T-shaped contraceptive tools designed to provide reliable, reversible, and long-acting pregnancy prevention. Emerging innovations, such as iron-based non-hormonal devices, are being developed to minimize side effects and provide hormone-free solutions. These technological advancements are transforming the IUD landscape, enhancing choices for women, and reinforcing long-term market expansion.
| Market Scope | |
|---|---|
| Start Year | 2024 |
| Forecast Year | 2025-2034 |
| Start Value | $4 Billion |
| Forecast Value | $11 Billion |
| CAGR | 10.9% |
The hormonal IUD segment held 53.8% share in 2024, supported by its effectiveness in preventing pregnancy and improving menstrual health. Hormonal IUDs, particularly those releasing levonorgestrel, are widely adopted for their long-lasting protection, low maintenance, and added therapeutic advantages, such as reduced menstrual bleeding and relief from endometriosis symptoms. Healthcare providers frequently recommend them due to their dual benefits, strengthening their dominance in the market.
The hospitals segment held 53.2% share in 2024 and is forecasted to generate USD 5.8 billion by 2034. Hospitals remain vital in delivering long-acting reversible contraception, as they provide access to skilled gynecologists and obstetricians for safe insertion procedures. The adoption of postpartum IUD insertion protocols in several regions has broadened access and contributed to a decline in unplanned pregnancies, particularly in areas with robust maternal healthcare systems.
U.S. Intrauterine Devices Market was valued at USD 1.44 billion in 2024 and is estimated to grow at a CAGR of 10.3% between 2025 and 2034. Increased public health initiatives and awareness campaigns have encouraged the use of IUDs as a safe, effective, and convenient contraceptive option. Growing preference for long-acting reversible contraception over short-term alternatives, such as daily pills, has further accelerated adoption across diverse demographics in the U.S. and Canada.
Prominent companies active in the Intrauterine Devices Industry include MONA LISA, Meril, Sebela Pharmaceuticals, DKT, PREGNA, SMB, AbbVie, Prosan, GIMA, Bayer, Medicines360, eurogine, GYNO CARE, CooperSurgical, HLL Lifecare Limited, and others. To strengthen their foothold in the intrauterine devices market, leading companies are focusing on expanding product portfolios with both hormonal and non-hormonal options, including the development of innovative materials designed to minimize side effects and improve user comfort. Strategic collaborations with healthcare organizations and government programs are being pursued to broaden access and adoption rates. Many players are also investing in awareness campaigns to educate women on the benefits of IUDs beyond contraception, such as menstrual health management.
Table of Contents
Chapter 1 Methodology and Scope
- 1.1 Market scope and definition
- 1.2 Research design
- 1.2.1 Research approach
- 1.2.2 Data collection methods
- 1.3 Data mining sources
- 1.3.1 Global
- 1.3.2 Regional/Country
- 1.4 Base estimates and calculations
- 1.4.1 Base year calculation
- 1.4.2 Key trends for market estimation
- 1.5 Primary research and validation
- 1.5.1 Primary sources
- 1.6 Forecast model
- 1.7 Research assumptions and limitations
Chapter 2 Executive Summary
- 2.1 Industry 3600 synopsis
- 2.2 Key market trends
- 2.2.1 Regional trends
- 2.2.2 Product trends
- 2.2.3 Age group trends
- 2.2.4 End use trends
- 2.3 CXO perspectives: Strategic imperatives
- 2.3.1 Key decision points for industry executives
- 2.3.2 Critical success factors for market players
- 2.4 Future outlook and strategic recommendations
Chapter 3 Industry Insights
- 3.1 Industry ecosystem analysis
- 3.2 Industry impact forces
- 3.2.1 Growth drivers
- 3.2.1.1 Favorable regulatory scenario
- 3.2.1.2 Rising awareness among women regarding various IUD applications
- 3.2.1.3 High number of unintended pregnancies
- 3.2.1.4 Government initiatives for the prevention of unwanted abortions and pregnancies
- 3.2.1.5 Growing inclination towards planned delayed pregnancy
- 3.2.2 Industry pitfalls and challenges
- 3.2.2.1 High cost of the device
- 3.2.2.2 Risk of several health issues
- 3.2.2.3 Variability in insurance coverage and access
- 3.2.3 Market opportunities
- 3.2.3.1 Rising demand for long-term contraception
- 3.2.1 Growth drivers
- 3.3 Growth potential analysis
- 3.4 Regulatory landscape
- 3.5 Technological landscape
- 3.5.1 Current technologies
- 3.5.2 Emerging technologies
- 3.6 Future market trends
- 3.7 Reimbursement scenario
- 3.8 Consumer behaviour and trends
- 3.9 Brand analysis
- 3.10 Pipeline analysis
- 3.11 Therapeutic applications beyond contraception
- 3.12 Pricing analysis, 2024
- 3.13 Porter's analysis
- 3.14 PESTEL analysis
- 3.15 Gap analysis
Chapter 4 Competitive Landscape, 2024
- 4.1 Introduction
- 4.2 Company matrix analysis
- 4.3 Company market share analysis
- 4.4 Competitive analysis of major market players
- 4.5 Competitive positioning matrix
- 4.6 Key developments
- 4.6.1 Mergers and acquisitions
- 4.6.2 Partnerships and collaborations
- 4.6.3 New product launches
- 4.6.4 Expansion plans
Chapter 5 Market Estimates and Forecast, By Product, 2021 - 2034 ($ Mn)
- 5.1 Key trends
- 5.2 Copper IUD
- 5.3 Hormonal IUD
Chapter 6 Market Estimates and Forecast, By Age Group, 2021 - 2034 ($ Mn)
- 6.1 Key trends
- 6.2 15-19
- 6.3 20-24
- 6.4 25-29
- 6.5 30-34
- 6.6 35-39
- 6.7 40-44
- 6.8 45+
Chapter 7 Market Estimates and Forecast, By End Use, 2021 - 2034 ($ Mn)
- 7.1 Key trends
- 7.2 Hospitals
- 7.3 Gynecology clinics
- 7.4 Community health care centers
Chapter 8 Market Estimates and Forecast, By Region, 2021 - 2034 ($ Mn)
- 8.1 Key trends
- 8.2 North America
- 8.2.1 U.S.
- 8.2.2 Canada
- 8.3 Europe
- 8.3.1 Germany
- 8.3.2 UK
- 8.3.3 France
- 8.3.4 Spain
- 8.3.5 Italy
- 8.3.6 Netherlands
- 8.4 Asia Pacific
- 8.4.1 China
- 8.4.2 Japan
- 8.4.3 India
- 8.4.4 Australia
- 8.4.5 South Korea
- 8.5 Latin America
- 8.5.1 Brazil
- 8.5.2 Mexico
- 8.5.3 Argentina
- 8.6 Middle East and Africa
- 8.6.1 South Africa
- 8.6.2 Saudi Arabia
- 8.6.3 UAE
Chapter 9 Company Profiles
- 9.1 AbbVie
- 9.2 Bayer
- 9.3 CooperSurgical
- 9.4 DKT
- 9.5 eurogine
- 9.6 GIMA
- 9.7 GYNO CARE
- 9.8 HLL Lifecare Limited
- 9.9 Medicines360
- 9.10 Meril
- 9.11 MONA LISA
- 9.12 PREGNA
- 9.13 Prosan
- 9.14 Sebela Pharmaceuticals
- 9.15 SMB




